Liquids, Solids and Changes of State
Physical Properties versus Chemical Properties
Although much chemistry is devoted to chemical properties and
reactions, the physical properties of substances often concern us more
on a day-to-day basis. Farmers worry about whether the expected
precipitation will come in liquid or solid form. Rain, a liquid, is
usually welcomed; hail, a solid, is almost universally dreaded because
of the damage it can inflict on crops. The fact that water expands when
it solidifies may bring disaster (or at least an expensive repair bill)
to the automobile owner who has neglected to put antifreeze in the car's
radiator if the temperature drops very far below freezing. During
summer, the high rate of evaporation of paint solvent can make painting
in the hot sun difficult. In Banff the traditional "three minute egg"
isn't nearly as well cooked as most people like, so it's boiled a little
longer.
These are just a few example that show how the physical
properties of substances and the transformations among the three states
of matter influence our lives. One of the goals of chemistry has been to
understand what determines these properties.
Why Gases Differ From Liquids and Solids
Physical properties depend on forces of attraction between
molecules, which are strong in liquids and solids and very weak in
gases.
Most of the physical properties of gases, liquids, and solids are
actually controlled by the strengths of intermolecular attractions - the
attractive forces that exist between neighbouring particles. In liquids
and solids, these forces are much stronger than in gases. But why?
Anyone who has ever played with magnets has discovered an
important property of attractive forces between things. Whether these
forces are magnetic or electrical, their strengths decrease quite
rapidly as the distance between the attracting particles increases. For
example, when two magnets are placed near each other, the attraction
between them can be quite strong, but if they are far apart hardly any
attraction is felt at all. This same phenomenon is what is primarily
responsible for that fact that gases have properties that depend very
little on chemical composition, whereas the properties of liquids and
solids are influenced by chemical composition to a very large extent.
In a gas the molecules are far apart. At these large distances,
the attractive forces are very weak, so the differences caused by
dissimilarities in chemical makeup are very small and are hardly
noticeable at all. As a result, all gases seem to be pretty much alike.
However, in a liquid or solid, where the molecules are very close to
each other, these attractive forces become quite strong. Differences
between the intermolecular attractions caused by variations in chemical
composition become magnified, and this causes unlike substances to
behave quite differently.
Intermolecular Attractions
Dipole-dipole forces, London forces, and hydrogen bonding are the
principle kinds of intermolecular attractions.
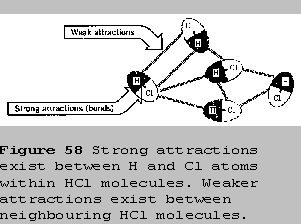
The attractions between molecules are always much weaker
than the attractions within molecules. In an molecule of HCl, the
hydrogen atom and chlorine atom are held to each other very tightly by a
covalent bond. Neighbouring HCl molecules are attracted to each other by
much weaker forces. Therefore, when a particular chlorine atom moves,
the hydrogen atom bonded to it is forced to go along, and the HCl
molecule remains intact as it moves about.
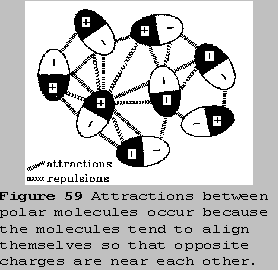
Dipole-Dipole Attractions
Dipole molecules like HCl tend to line up so that the positive
end of one dipole is near the negative end of another. Thermal energy,
however, causes the molecules to jiggle about, so the alignment isn't
perfect. Nevertheless, there is still a net attraction between the polar
molecules. These attractive forces are called dipole-dipole attractions.
Generally they are only about 1% as strong as a covalent bond, and their
strengths decrease quite rapidly as the distance between molecules
increases.
London Forces
It is fairly easy to understand why there are attractions between
polar molecules such as HCl or SO2. But even between the
particles of nonpolar substances such as Cl2 or CH4,
there are attractive forces. Both Cl2 and CH4 can
be condensed to liquids and even solids if cooled to low enough
temperatures, so there must be attractions between their particles that
cause them to cling together in the liquid or solid state. These
attractions between nonpolar particles are considerably weaker then
those of polar molecules.
Take a look at the table below for two polar and two nonpolar
substances.
Substance Boiling Point Formula weight Number of Electrons
Polar
HCl -84.9 36
18
H2S -60.7
34 18
Non-polar
F2 -188.1
38 18
Ar -185.7 40
18
All four are somewhat similar in that they have similar formula
weights and the same number of electrons. Notice that the two nonpolar
substances have very similar boiling points. Notice also that the two
polar compounds have similar boiling points. However, the nonpolar
substances have boiling points that are at least 100 degrees lower then
those of the polar substances, which tells us that the attractions in
the nonpolar liquids are considerably weaker then those in the polar
liquids. They only become noticeable when the thermal energy is low
enough for the molecules to slow down so that the forces can work.
In 1930, Fritz London, a German physicist, offered a simple
explanation for the weak attractions between nonpolar particles. In any
atom or molecule the electrons are constantly moving, and if we could
examine this motion in two neighbouring particles, we would find that
the movement of electrons in one particle influences the movement of
electrons in the other.
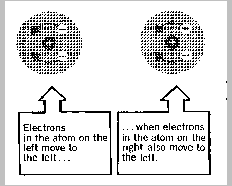
This is because electrons repel each other and tend to stay as
far apart as then can. Therefore, as an electron of one particle gets
near the other particle, electrons on the second are pushed away. This
happens continually as the electrons move around, so to some extent, the
electron density in both particles flickers back and forth in a
synchronous fashion. Take a look at the series of instantaneous "frozen"
views below.
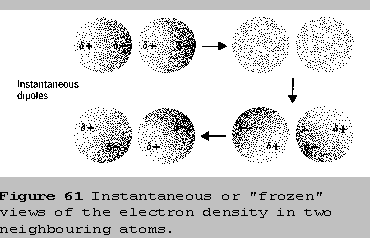
Notice that at any given moment the electron density of a
particle can be unsymmetrical, with more negative charge on one side
than on the other. For that particular instant, the particle becomes a
dipole, and we call it a momentary dipole, or an instantaneous dipole.
As the instantaneous dipole forms in one particle, it pushes
electron density around in its neighbour, which causes the electron
density there to become unsymmetrical, too. As a result, this second
particle also becomes a dipole, and we call it an induced dipole because
it is caused by, or induced by, the formation of the first
dipole. Since the electrons are in constant motion these dipoles flicker
into existence then vanish just as quickly. Moments later the dipoles
reappear in a different orientation and there will be another brief
dipole-dipole attraction. These momentary dipole-dipole attractions tend
to be very very weak because they are only "turned on" part of the time.
The momentary dipole-dipole attractions are called
instantaneous dipole-induced dipole attractions, or London forces,
to distinguish them from the kind of dipole-dipole attractions that
exist in polar molecules.
London forces are the only kind of attraction present between
nonpolar molecules. They are also present between polar molecules and
they even occur in ions. However, in polar molecules and ions these
London forces contribute relatively little to the overall attractions
between polar molecules and ions.
The strengths of London forces depend on several factors. One of
them is the size of the electron cloud of the particle. In general, when
the electron cloud is large, the outer electrons are not held very
tightly by the nucleus. This makes the electron cloud "mushy" and rather
easily deformed, which is precisely the condition that most favours the
formation of an instanteous dipole or the creation of an induced dipole.
Nuclei with large electron clouds therefore form short-lived dipoles
more easily and experience stronger London forces than do similar nuclei
with small electron clouds. The effects of size can be seen if you
compare the boiling points of the halogens or the noble gases. Those
that are large, have higher boiling points (and therefore stronger
intermolecular attractions) then those that are small.
Group VIIA Boiling Point(oC) Group O Boiling Point (oC)
F2 -188.1
He -268.6
Cl2 -34.6
Ne -245.9
Br2 58.8
Ar -185.7
I2 184.4
Kr -152.3
Xe
-107.1
Rn
-61.8
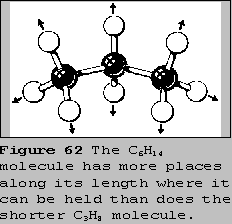
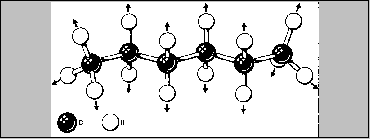
A second factor that affects the strength of London forces is the
number of atoms in a molecule. For molecules containing the same
elements, the London forces increase with the number of atoms. The
hydrocarbons are a good example. Take a look again at the alkane series
and their boiling points in your databook. As the molecular mass
increases by CH2 the boiling points also increase. This means
that the molecule with the longer chain length experiences the stronger
attractive forces. This is so because it has more places along its
length where it can be attracted to other molecules. Even if the
strength of attraction at each location is about the same, the total
attraction experienced by the longer molecules is greater.
Hydrogen Bonds
These are a special case of dipole-dipole attraction. When
hydrogen is covalently bonded to a very small, highly electronegative
atom such as fluorine, oxygen, or nitrogen, unusually strong
dipole-dipole attractions are often observed. There are two reasons for
this. First, because of the large electronegativity difference, the F-H,
O-H, or N-H bonds are very polar. The ends of these dipoles carry a
substantial portion of one charge. Second, because of the small size of
the atoms involved, the charge on the end of a dipole is also highly
concentrated. This makes it particularly effective at attracting the
opposite charge on the end of a neighbouring dipole. These two factors
combine to produce attractions, called hydrogen bonds, that are about
five times stronger than normal dipole-dipole attractions. Many
biological molecules such as DNA and protein depend upon their shape
because of these hydrogen bonds. Any factor that affects the shape of
these molecules usually does so by disrupting the hydrogen bonds.
Cooking an egg does this very nicely. Egg albumin is a soluble protein.
Cooking it disrupts all the hydrogen bonds which reform entirely at
random. The hard egg white that you eat is disrupted egg albumin
protein.
|

