Construction and use of the McCabe-Thiele diagram
Before starting the construction and use of a McCabe-Thiele diagram for the
distillation of a binary feed, the
vapor-liquid equilibrium (VLE) data must be obtained for the lower-boiling
component of the feed.
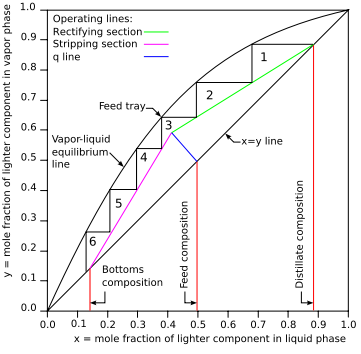
Figure 1: Typical McCabe-Thiele diagram for distillation of a binary
feed
The first step is to draw equal sized vertical and horizontal axes of a
graph. The horizontal axis will be for the mole fraction (denoted by x) of the
lower-boiling feed component in the liquid phase. The vertical axis will be for
the mole fraction (denoted by y) of the lower-boiling feed component in the
vapor phase.
The next step is to draw a straight line from the origin of the graph to the
point where x and y both equal 1.0, which is the x = y line in Figure 1. Then
draw the equilibrium line using the VLE data points of the lower boiling
component, representing the equilibrium vapor phase compositions for each value
of liquid phase composition. Also draw vertical lines from the horizontal axis
up to the x = y line for the feed and for the desired compositions of the top
distillate product and the corresponding bottoms product (shown in red in Figure
1).
The next step is to draw the operating line for the rectifying section (the
section above the feed inlet) of the distillation column, (shown in green in
Figure 1). Starting at the intersection of the distillate composition line and
the x = y line, draw the rectifying operating line at a downward slope (Δy/Δx)
of L / (D + L) where L is the molar flow rate of
reflux and D is
the molar flow rate of the distillate product. For example, in Figure 1,
assuming the molar flow rate of the reflux L is 1000 moles per hour and the
molar flow rate of the distillate D is 590 moles per hour, then the downward
slope of the rectifying operating line is 1000 / (590 + 1000) = 0.63 which means
that the y-coordinate of any point on the line decreases 0.63 units for each
unit that the x-coordinate decreases.
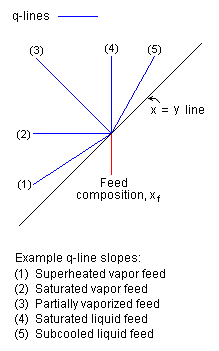
Examples of q-line slopes
The next step is to draw the blue q-line (seen in Figure 1) from the x = y
line so that it intersects the rectifying operating line.
The parameter q is the mole fraction of liquid in the feed and the slope of
the q-line is q / (q - 1). For example, if the feed is a saturated liquid it has
no vapor, thus q = 1 and the slope of the q-line is infinite which means the
line is vertical. As another example, if the feed is all saturated vapor, q = 0
and the slope of the q-line is 0 which means that the line is horizontal.
Some example q-line slopes are presented in Figure 2. As can be seen now, the
typical McCabe-Thiele diagram in Figure 1 uses a q-line representing a partially
vaporized feed.
Next, as shown in Figure 1, draw the purple operating line for the stripping
section of the distillation column (i.e., the section below the feed inlet).
Starting at the intersection of the red bottoms composition line and the x = y
line, draw the stripping section operating line up to the point where the blue
q-line intersects the green operating line of the rectifying section operating
line.
Finally, as exemplified in Figure 1, draw the steps between operating lines
and the equilibrium line and then count them. Those steps represent the
theoretical plates (or equilibrium stages). The required number of
theoretical plates is 6 for the binary distillation depicted in Figure 1.
Note that using colored lines is not required and only used here to make the
methodology easier to describe.
In
continuous distillation with varying
reflux ratio,
the
mole fraction of the lighter component in the top part of the
distillation column will decrease as the
reflux ratio
decreases. Each new
reflux ratio will alter the
slope of the
rectifying section operating line.
When the assumption of constant molar overflow is not valid, the operating
lines will not be straight. Using mass and enthalpy balances in addition to
vapor-liquid equilibrium data and enthalpy-concentration data, operating lines
can be constructed based on Ponchon-Savarit's method.

