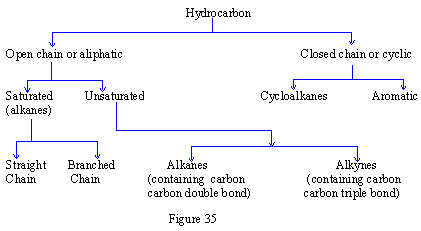
Following are some of the examples of hydrocarbons:
Homologous series :
A series of compounds which have a common general
formula and in which each member differs from the next member by a constant
unit, which is the methylene group (-
CH2-) is
called the homologous series. Members of a homologous series are called
homologs.
A few examples of the homologous series with some of their homologs are given
below.
1) Straight chain alkanes having general formula CnH2n
+ 2
| Structure
|
Name |
| 1. CH4 |
Methane |
| 2. CH3 -
CH3 |
Ethane |
| 3. CH2-CH2-CH3 |
Propane |
| 4. CH3-CH2CH2CH3 |
Butane |
| 5. CH3-(CH2)3-CH3 |
Pentane |
| 6. CH3-(CH2)4-CH3 |
� Hexane |
| 7. CH3(CH2)5CH3 |
� Heptane |
| 8. CH3
(CH2)6
CH3 |
� Octane |
| 9. CH3
(CH2)7CH3 |
� Nonane |
| 10. CH3
(CH2)8 CH3 |
n - Decane |
2) Alcohols having the general formula
CnH 2n + 1OH
| CH3-OH |
Methanol |
| CH3-CH2-OH |
Ethanol |
| CH3(CH2)2OH |
n-Propanol |
| CH3 (CH2)3OH |
n- butanol |
3) Amines having the general formula CnH2n + 1NH2
| CH3-NH2 |
Methylamine |
| CH3
CH2
NH2 |
Ethylamine |
| CH3(CH2)2
NH2 |
n-Propylamine |
| CH3(CH2)3NH2 |
n-Butylamine |
General characteristics of alkanes of a homologous series.
1. Lower members are gases.
2. Members with five to sixteen carbon atoms are liquid.
3. Higher members are solids.
4. Density increases with increase in molecular weight.
5. Boiling point increases with increase in molecular weight.
6. Melting point increases with increase in molecular weight.
7. Insoluble in water but soluble in organic solvents.
8. Exhibit isomerism.