Atomic Weights of An Elements
The atomic weight of an element is defined as a number which expresses
the ratio of weight of one atom of an element to the weight of one atom of
hydrogen.

For e.g. The atomic weight of nitrogen is 14 which implies that an atom of
nitrogen is 14 times heavier than one atom of hydrogen.
In recent times, the naturally occurring isotope of carbon C12 is
taken as standard.
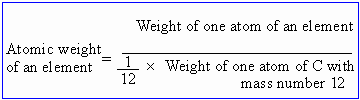
Thus atomic weight is only a number. It has no units and it does not provide
any information regarding the actual weight of an atom of an element.
Fractional Atomic Weight :
A large number of naturally occurring elements consists of a mixture of
isotopes (atoms of the same elements with the same atomic number but different
atomic masses). The properties of these isotopes are constant and hence an
element has a fixed atomic weight. The atomic weight of an element thus
represents the average of atomic masses of different isotopes of the element.
This in some cases leads to fractional atomic weight.
For e.g. Chlorine possesses two isotopes with atomic masses 35 & 37 in the
proportion of 3 : 1.
Hence,
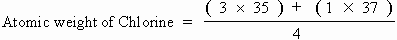
= 35.5
Gram atomic weight :
The atomic weight of an element expressed in grams is known as gram atomic
weight or 1 gm. atom of an element.
e.g. 1 gm. atom of Bromine = 79.9 gms. of Bromine
1 gm. atom of Sodium = 22.98 gms of Sodium
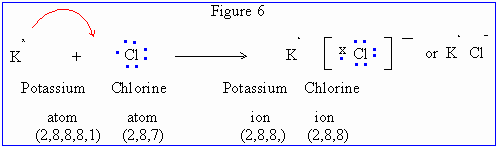
At a glance
|