Concentration of Solution
Concentration of Solutions can be expressed in different ways as
follows :
i)Weight percentage of solute is the weight of solute
(� 100), divided by the total weight of the solution.
i.e. 10% w/w of NaCl means 10 gms of NaCl is present in a 100 g of solution.
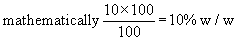
ii) The molarity, M, of a solution is the number of moles of
solute per kg of solvent.
20 M of sodium chloride solution means 20 moles (20 �
55.5 gms) of sodium chloride dissolved in a solvent to get one liter of
solution.
iii) The molality, m, of a solution is the number of moles of solute
per kg of solution.
12 m of sodium chloride solution means 12 moles (12 �
55.5 gms) of sodium chloride dissolved in a solvent to get 1 kg of
solution.
iv) The normality, N, of a solution is the number of gram equivalent
weight of solute per liter of solution.
5 N of sulphuric acid solution means ( 5 �
49 gms ) ( \ eq. wt = mol.wt / no.of
equivalents = 98/2 = 49 ) of sulphuric acid dissolved in solvent to
get one liter of solution.
v) The mole fraction, X, is the ratio of the number of moles of solute
( or solvent ) to the total number of moles of all components in a solution.
In a 10% HCl solution in H2O
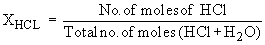
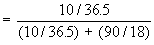
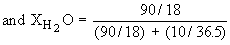
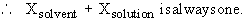
At a glance the concentration of solutions:
|
No. |
Name |
Symbol |
Explanation |
|
1 |
Weight percentage |
% w/w |
Weight of solute in percentage in 100 gm of
solution. |
|
2 |
Molarity |
M |
Number of moles of solute per liter of
solution. |
|
3 |
Molality |
m |
Number of moles of solute per kg of
solution. |
|
4 |
Normality |
N |
Number of gram equivalent weights of solute
per liter of solution. |
|
5 |
Mole fraction |
X |
Ratio of the number of moles of solute (or
solvent) to the total number of moles of all components in a solution. |
|