Effect of Temperature and Pressure on Solubility
When the solute dissolves with the absorption of heat
energy + solute + H2O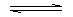 saturated solution.
saturated solution.
e.g. sodium chloride, potassium bromide.
When the solute dissolves with the evolution of heat
solute + H2O
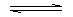 saturated
solution + energy saturated
solution + energy
e.g. Lithium carbonate, sodium sulphate
Thus the solubility of a solute where energy is required (former
example) to shift the equilibrium to the right side, increases with increasing
temperature of the solution and decreases with decreasing temperature of the
solution.
| The solubility of a solute where
energy is liberated (latter example) during the shifting of equilibrium
to the right side, increase in temperature decreases the solubility and
by decreasing the temperature solubility increases. |
The solubility of all the gases decreases with increase in temperature. Thus
when the temperature of soft drinks is raised CO2
gas evolves as its solubility decreases in the soft drink solution.
The solubility of solid and liquid solutes have negligible effect on the
pressure of a system.
But the solubility of a gas is directly proportional to the system pressure.
|