Electrochemical Cells
Now let us learn about cells which generate electricity. A device
which generates electrical energy from chemical energy is called an
Electrochemical cell. e.g. transistor cell ( dry cell )
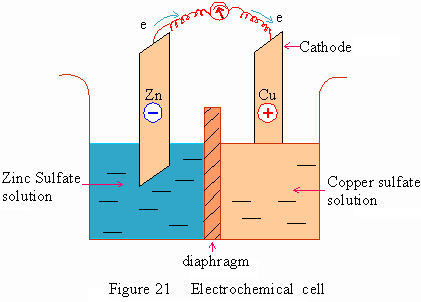
A Zinc rod is placed in Zinc sulfate solution separated by a diaphragm from
the copper sulfate solution placed with a copper rod. An electron flow is
produced by spontaneous oxidation-reduction reactions taking place at the
electrodes.
Reaction :
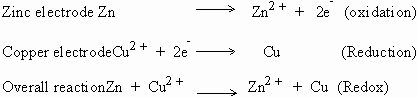
The electrode at which oxidation takes place is called the positive (+)
electrode and conversely the reduction electrode is termed as the
negative (-) electrode.
Types of Electrodes
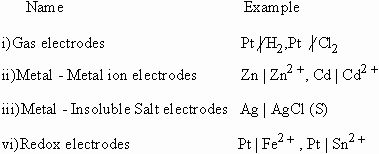
|