Factors Affecting Chemical Equilibrium
1) Effect of change of concentration
By increasing or decreasing the concentration of one of the reactants or
products, the reaction can be manipulated in the desired direction.
To increase the yield of NH3 the concentration of N2
or preferably H2 should be increased in the synthesis of
ammonia.
2) Effect of change of pressure
Reactions which proceed with decrease in volume are favored by high
pressures.
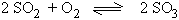
2 vol 1 vol 2 vol
On the other hand, reactions which proceed with increase in volume are
favored by low pressures.
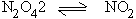
1 vol 2 vol
If the volume remains the same then change in pressure has no effect.
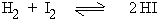
1 vol 1 vol 2 vol
3) Effect of change in temperature
Exothermic reactions will be favored by decrease in temperature
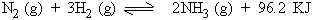
Endothermic reactions will be favored by increase in temperature.
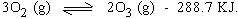
4) Effect of catalyst
The presence of a catalyst in a reversible reaction does not affect the state
of equilibrium but only causes the reaction system to attain equilibrium in a
shorter period of time.
|