Grahamís Law of Diffusion
The phenomenon of diffusion of a gas can be defined as the tendency of
a gas to spread uniformly throughout the space available to it.
The relation between its density and the rate of diffusion of a gas can be
represented by Grahamís law which states that "The rate of diffusion of a
gas is inversely proportional to the square root of its density under given
conditions of temperature and pressure."
It can be mathematically expressed as

If r1 & r2 are rates of diffusion with densities d1
& d2 the above equation becomes,
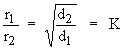
Vapor density of a gas can be calculated if 0.08 dm3 of gas
diffuses in the same time as 0.002 dm3 of chlorine having vapor density 35.5.
r1 = rate of diffusion of gas = 0.08 dm3/t
r2 = rate of diffusion of chlorine = 0.002 dm3/t
D1 = ?
D2 = 35.5
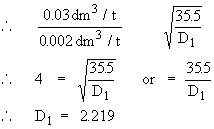
|