Isotopes
Different atoms of the same element possessing different atomic masses but
having same atomic number are known as Isotopes. Since the isotopic atoms
have the same atomic number, they must contain an equal number of protons.
As
their atomic masses are different, there must be a difference in the number of
neutrons they possess. They will show similar chemical properties but their
physical properties will be different due to different masses. Also since all
isotopes of the same element have the same atomic number they occupy the same
place in the periodic table.
Fractional atomic masses can be explained from the study of isotopes.
Chlorine has two major isotopes having atomic masses 35 and 37 and present in
the ratio 3 : 1. Hence atomic weight of chlorine is neither 35 or 37 but the
average of the two.
i.e. atomic weight of chlorine =
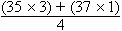
= 35.5
Examples of isotopes of some common elements are illustrated in following
table.
|
Table 3 Isotopic Elements |
|
Element |
Atomic No. |
Mass No. |
Symbol |
No. of Protons |
No. of Neutrons |
Abudance |
|
Hydrogen
Deuterium
Tritium |
1
1
1 |
1.0078
2.0141
3.0161 |
1H1
1D2
1T3 |
1
1
1 |
0
1
2 |
99.99%
0.02%
rare |
|
Oxygen |
8
8
8 |
16
17
18 |
8O16
8O17
8O18 |
8
8
8 |
8
9
10 |
99.76%
0.04%
0.20% |
|
Chlorine |
17
17 |
34.97
36.97 |
17C135
17C137 |
17
17 |
18
20 |
76%
26% |
|
Neon |
16
16 |
20
22 |
16Ne20
16Ne22 |
16
16 |
4
6 |
90%
10% |
The above table can be used in the following way to find out the atomic
weight of naturally occurring elements.
Example :
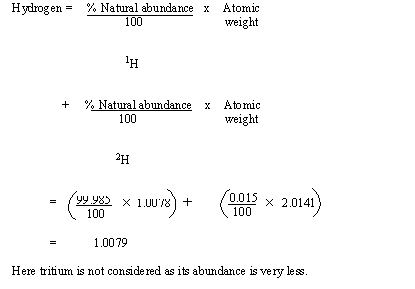
Here tritium is not considered as its abundance is very less.
|