Le-Chatelierís Principle
Once an equilibrium is established, no further change is apparent as long as
the external conditions remain unchanged.
On changing the external conditions, whether the equilibrium will shift
towards the reactants or the products can be predicted by the application of
Le-Chatelierís principle which states that
When a stress (change in concentration, temperature or total pressure) is
applied to a system at equilibrium, the system readjusts so as to relieve or
offset the stress.
Application of Le-Chatelierís principle
In the synthesis of Ammonia (Haberís process)
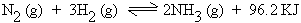
1 vol 3 vol 2 vol
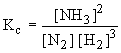
|