Polar Bonds
Polar Bonds are formed when one of the elements attracts the shared
electrons more strongly than the second element. These bonds are somewhere
between covalent and pure ionic bonds in character. There is asymmetry in the
distribution of electron density. Usually in common expression we speak of
electrons being shifted towards the more electronegative partner.
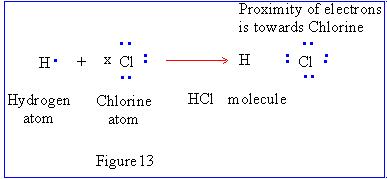
The unequal sharing of electrons leads to fractional
electrical charges on atoms represented by the Greek letter delta

|