Polyprotic Acids
Polyprotic acids can be defined as those acids that contain more than one
acid hydrogen (proton) per molecule.
e.g. H2SO4,
Oxalic acid (H2C2O4),
H3PO4,
arsenic acid (H3HSO4)
These acids ionize in a step wise manner (number of steps depends on number
of acid hydrogen) and there is an ionization constant for each step.
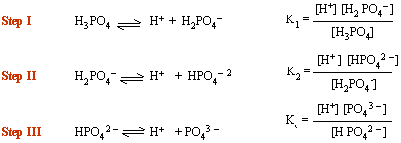
In all polyprotic acids
K1 > K2
> K3
i.e. primary ionization is stronger than secondary which is in turn stronger
than tertiary.
The ionization constant of some polyprotic acids are given in the following
table:
|
Table 19
Arsenic H3AsO4
H+ + H2AsO4-
K1= 2.5 � 10- 4
H2AsO4-
H+ + HAsO42
- K2=5.6 � 10- 8
H3AsO42
- H+ + AsO43
- K3=3 �10- 13
Oxalic H2C2O4 H+ + HC2O4-
K1=5.9 � 10- 2
H2C2O4-
H+ + C2O42 - K2=6.4 � 10- 5
Phosphoric H3PO4 H+ + H2PO4-
K1=7.5 � 10- 3
H2PO4- H+
+ HPO42 - K2=6.2 � 10- 8
HPO42 - H+ +
PO43 - K3=1 � 10- 12
Sulfuric H2SO4 H+ + HSO4-
K1=Strong
HSO4- H+ + SO42 -
K2=1.3 � 10- 2 |
As the rate of dissociation for successive dissociating is very low [ H+
] from those is (almost negligible) in comparison, hence pH of the solution is
determined mainly by first dissociation (principle source of primary H+
).
Consider the following equilibria to calculate ion concentrations in 0.01 m
solution of H2S.
(K1 = 1.1 � 10- 7, K2 = 1 � 10- 14)
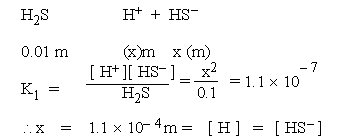
Applying these to second dissociation
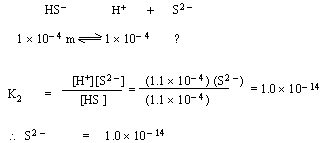
Polyprotic acids form more than one salt. Depending upon the
molar ratio of reactants, the reaction of NaOH & H2SO4 yields either
Na2SO4 (sodium sulphate) or the acid salt NaHSO4 (sodium hydrogen
sulphate).
|