CHEMICAL EQUILIBRIUM
Many physical and chemical changes which we encounter are reversible. For
e.g. water changes into ice when temperature is lowered; increasing the
temperature reverses this process. Conversely, on heating water, it converts
into steam; lowering its temperature reverses the process.
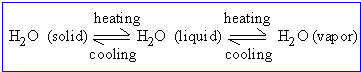
When the conditions are such that forward ( represented by
 )
and backward (represented by )
and backward (represented by )reactions
can both occur to a noticeable extent, the process is described as a
reversible reaction. )reactions
can both occur to a noticeable extent, the process is described as a
reversible reaction.
It has been found that after a certain time interval, reversible reactions
attain a state of chemical equilibrium i.e. a state in which no further change
in composition with time can be detected, provided the temperature and pressure
are not altered.
11.1 Types of Reactions
a) Reversible reactions
Reactions in which products themselves react (or decompose) to produce the
starting reactants, are termed as reversible reactions. Such reactions are
denoted by the use of two half arrows.
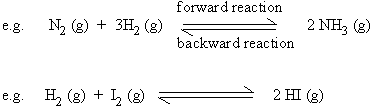
For a reverse reaction to occur, the reaction should be carried out in a
closed container when one of the products is gaseous.
b) Irreversible reactions
The reactions in which products do not recombine to form the original
reactants is called an irreversible reaction.
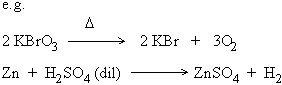
c) Exothermic reactions
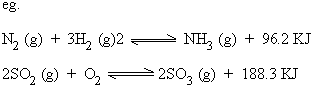 It is a chemical reaction which proceeds with the liberation of heat energy.
It is a chemical reaction which proceeds with the liberation of heat energy.
d) Endothermic reactions
It is a chemical reaction which proceeds with the absorption of heat energy.
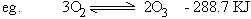
Chemical Equilibrium
Consider a reversible homogenous reaction occurring in a closed container
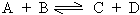
Initially the molar concentration of 'A' & 'B' being maximum the rate of
forward reaction is maximum. As the reaction proceeds 'C' & 'D' appear in the
reaction and the backward reaction sets in but at much lower rate.
The changing rates of forward and backward reactions with time can be
represented as follows :
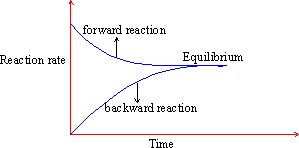
Figure 22 Equilibrium graph
|