Acids and bases are used throughout
chemistry.
Definitions of acids and bases
There are three common definitions of acids and bases:
- Arrhenius acid: Produces hydronium ion
in water.
- Arrhenius base: Produces hydroxide ion
in water.
- Br�nsted-Lowry acid: Donates a proton
(H+1)
- Br�nsted-Lowry base: Accepts a proton
- Lewis acid: Accepts electrons
- Lewis base: Donates electrons
Strong acids and bases are ones in which most
molecules perform their �duty� while weak acids and bases only have
a few acid and base molecules that act as acids and bases. There
are only a few strong acids and bases to remember�the rest will most
likely be weak. Strong acids: HCl, HBr, HI, HNO3, HClO3, HClO4.
Strong bases: NaOH, KOH, Ca(OH)2, Ba(OH)2, Sr(OH)2
A conjugate acid is what remains after a base
does its �job�. A conjugate base is what�s left after an acid does
it�s �job.� Strong acids or bases form a weak conjugate and vice
versa.
pH
The pH scale is a logarithmic scale to measure the acidity of a
solution.
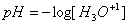 Strong
acids and bases can be assumed to dissociate completely. Therefore,
the concentration of the original strong acid or base is assumed to
equal the concentration of the hydrogen or hydroxide ion. For weak
acids or bases, the equilibrium constant and ICE charts are used to
determine the concentration of the hydronium ion before solving for
pH.
Strong
acids and bases can be assumed to dissociate completely. Therefore,
the concentration of the original strong acid or base is assumed to
equal the concentration of the hydrogen or hydroxide ion. For weak
acids or bases, the equilibrium constant and ICE charts are used to
determine the concentration of the hydronium ion before solving for
pH.
Acid/base properties of salts
Some salts can have acid/base properties based on the acid or base
they are based off of.
Salts from
- Weak acid + strong base = Basic
- Weak acid + weak base = Neutral
- Strong acid + weak base = Acidic
- Strong acid + Strong base = Neutral
Buffers
A buffer is a solution containing a weak acid and its conjugate base
or a weak base and its conjugate acid. Buffers resist changes in pH
when acids or bases are added. The pH will still change, but much
less than if it was plain water.
Titrations
Titrations are used to find the concentration of an unknown solution
using a solution of known concentration. An indicator is used that
changes color at the stoichiometric point (the point at which no
reactants are left over) based on the pH of the products that are in
solution at that point. Stoichiometry is used at that point to
determine the unknown concentration.