Antiamoebic drugs
Abstract
Background
Amoebiasis is a major public health problem in tropical and
subtropical countries. Although a number of antiamoebic agents are used for its
treatment, yet the susceptibility data on clinical isolates of Entamoeba
histolytica and Entamoeba dispar are not available. Therefore, the
present study was aimed to assess the in vitro susceptibility of clinical
isolates of E. histolytica and E. dispar to metronidazole,
chloroquine, emetine and tinidazole.
Methods
A total of 45 clinical isolates (15 E. histolytica and
30 E. dispar) were maintained in polyxenic cultures followed by monoxenic
cultures. In vitro drug sensitivity (IC50) of clinical isolates and
standard reference strain of E. histolytica (HM1: IMSS) was assessed by
nitro blue tetrazolium (NBT) reduction assay after exposure to various
concentrations of each drug.
Results
The results showed that all clinical isolates had a higher IC50
compared to reference strain to all the four drugs. E. histolytica
isolates appeared to be more susceptible [IC50 (hormone-μm)
13.2,26.3,31.2 and 12.4] compared to E. dispar isolates [IC50(hormone-μm)
15.6,28.9,32.8 and 13.2] and the reference strain of E. histolytica [IC50
(hormone-μm) 9.5, 15.5, 29.9 and 10.2] to the metronidazole,
chloroquine, emetine and tinidazole respectively.
Conclusions
The results indicate that till date, Entamoeba isolates
in India do not seem to be resistant to the commonly used Antiamoebic drugs.
Background
Entamoeba
histolytica, is the etiological agent of amoebic dysentery and amoebic liver
abscess (ALA). Worldwide, 40-50 million symptomatic cases of amoebiasis occur
annually and 70,000 to 100,000 deaths due to this infection .
There are two distinct, but morphologically identical species of Entamoeba:
Entamoeba histolytica, which is pathogenic and Entamoeba dispar,
which is non-pathogenic. E. histolytica, has the capacity to invade
intestinal mucosa resulting in intestinal amoebiasis and cause extra intestinal
amoebiasis [amoebic liver abscess (ALA)] .
Infection is primarily treated by instituting antiamoebic
therapy. Drugs of choice for invasive amoebiasis are tissue active agents, like
metronidazole, tinidazole and chloroquine or the more toxic emetine derivatives,
including dehydroemetine. Metronidazole and tinidazole are derived from
5-nitroimdazole which kill the trophozoites by alterations in the protoplasmic
organelles of the amoeba, but are ineffective in the treatment of cyst passers.
Chloroquine is derived from 4-aminoquinolines, which acts on the vegetative
forms of the parasite and kills it by inhibiting DNA synthesis. Emetine, a plant
alkaloid, kills the trophozoites of E. histolytica mainly by inhibiting
protein synthesis.
Indiscriminate use of drugs has led to an increase in the
minimum inhibitory concentration (MIC) of these therapeutic agents .
Although, drug resistance to E. histolytica does not appear to be a
serious problem, there are occasional reports of failure with metronidazole
suggesting that this could probably be heralding the development of drug
resistance clinically .
Recurrence of ALA even after treatment with metronidazole has been reported and
parasites may survive in spite of adequate treatment .
However, differences in drug sensitivity between strains of E. histolytica
have been reported, indicating that there may be a small percentage of amoebae
which are either resistant to the drug or may even eventually become resistant
due to abuse of antiamoebic agents .
Although, earlier studies have been focused on in vitro sensitivity of the only
axenic strains of E. histolytica ,
yet to the best of our knowledge, studies on in vitro drug susceptibility
studies on clinical isolates of E. histolytica and E. dispar have
not been reported. Therefore, in the present study an attempt has been made to
assess the in vitro activity of Antiamoebic drugs (emetine, chloroquine,
metronidazole and tinidazole) against clinical isolates of E. histolytica
and E. dispar.
Methods
Clinical isolates
Forty-five isolates from patients attending the Out Patient
Departments of Nehru hospital, attached to the Post Graduate Institute of
Medical Education & Research, Chandigarh, India, identified earlier
as either E. histolytica (15) or E. dispar (30) by hexokinase
isoenzyme analysis and by Techlab ELISA were used in the present study. These
have been cultured in modified Boeck and Drbohlav (NIH) medium
followed by Robinson's medium .
Preparation of antimicrobial agents
The drugs (metronidazole, chloroquine, emetine dihydrochloride
and tinidazole) used in the study were procured as pure salt from Sigma-Aldrich
Co., St. Louis, MO., 63178 USA. The stock solutions of drugs (each 0.1 M) were
prepared in dimethyl sulphoxide (DMSO)
and stored at -20�C till use. The stock solutions were diluted in medium to the
required concentration. A starting concentration used was 200
hormone-μM, which yielded a maximum concentration in the
assay of 17.1 hormone-μg/ml metronidazole, 51.59
hormone-μg/ml chloroquine, 55.3 hormone-μg/ml
emetine, and 24.7 hormone-μg/ml tinidazole.
In vitro drug sensitivity assay
Drug sensitivity to all the compounds was carried out by
nitroblue tetrazolium (NBT) reduction method .
Each clinical isolate was tested in duplicate along with the reference E.
histolytica strain (HM1: IMSS). Amoebae were harvested from 24 hour old
cultures and suspended in medium. The parasite count was adjusted to 3 � 105
parasites/ml in medium by haemocytometer .
The assay was carried out in microtiter plates (Grenier
bio-one, Germany). Briefly, in row A 200 hormone-μl of drug
and in all other rows (B-H) medium was added and doubling dilutions of the drug
were performed down the plate. Final drug concentration in rows A-H was as
follows: 100, 50, 25, 12.5, 6.25, 3.12, 1.6 and 0.8 (hormone-μM).
Further 100 hormone-μl of parasite suspension (3 � 105/well)
was added to all the rows (A-H). Each test included control (without drug) and
blank wells (medium only). The plates were incubated at 37�C for 4 hrs. The
contents of the plates were discarded and washed with pre warmed Hank's balanced
salt solution (HBSS pH 7.2). Thereafter, 100 hormone-μl of
NBT/well in HBSS was added and the plates were incubated at 37�C for 45 min.
followed by aspiration of the contents. Plates were then washed with HBSS twice
and 200 hormone-μl/well of DMSO (100% v/v) was added.
Following incubation at 37�C for 10 min, the optical density (OD) was measured
in an ELISA recorder at 540 nm.
The percentage of non-viable organisms, which failed to
metabolize NBT and therefore did not produce the dark blue formazan product, was
determined by applying the following formula:
Percentage of non-viable organisms at each drug conc. =
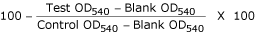
Statistical analysis
The mean IC50 values of all clinical isolates
against the four drugs were compared with corresponding IC50 values
of the reference E. histolytica strain (HM1: IMSS). Standard deviation
(SD) was used to indicate the extent of variation around group mean values. The
p value was calculated using the student's-t test.
Results
The IC50
values of emetine, chloroquine, metronidazole and tinidazole for the 45 clinical
isolates [15 E. histolytica and 30 E. dispar] and the reference
strain HM1: IMSS were determined by the NBT reduction assay. The mean IC50
values were significantly higher (P < 0.001) in E. dispar isolates
to all the four Antiamoebic drugs as compared to the E. histolytica
isolates and the reference E. histolytica strain
Discussion
Treatment failure
among amoebiasis patients often raises the possibility of drug resistance .
In the present study the 15 E. histolytica and 30 E. dispar
clinical isolates maintained by in vitro cultivation in monoxenic medium were
subjected to drug susceptibility tests against four Antiamoebic drugs:
metronidazole, chloroquine, emetine and tinidazole by NBT reduction assay. E.
histolytica reference strain (HM1: IMSS) was also included in each set of
experiments.
Results showed a significant difference in drug sensitivity in
clinical isolates as compared to the reference strain with all the four drugs.
The mean IC50 values (hormone-μm) of the E.
histolytica/E. dispar isolates against metronidazole,
chloroquine, emetine and tinidazole were 13.2/15.6, 26.3/28.9, 31.2/32.8 and
12.4/13.2 respectively. The IC50 values (hormone-μm)
of the reference strain against all the four respective drugs were 9.5, 15.5,
29.9 and 10.2. Recently Upcroft & Upcroft
have reported that the MIC values of metronidazole ranges from 12.5-25
hormone-μm for laboratory-passaged E. histolytica
strains. Adagu, et.al.
have shown the mean metronidazole IC50 value as 18.47
hormone-μm for the most susceptible isolates of E.
histolytica with a > 30 hormone-μm value as the cut off
for resistance. Burchard & Mirelman, studied in vitro sensitivity to
metronidazole and emetine of non-pathogenic zymodemes and showed that all were
similarly sensitive to both the drugs (1-10 hormone-μg/ml) .
In the present study, clinical isolates maintained in monoxenic culture were
used to detect the in vitro sensitivity as earlier it has been concluded that
bacterial flora associated with the amebae did not significantly interfere with
the test performance and sensitivity values .
Although resistance to metronidazole has been reported against
Trichomonas vaginalis ,
Giardia lamblia
and Leishmania donovani ,
yet to the best of our knowledge there is no documented resistance among
clinical isolates of E. histolytica and E. dispar.
Conclusion
The results of the
present study are in agreement with previous findings ,
except that there was a significantly higher IC50 value of all four
drugs to the clinical isolates as compared to the reference strain. E. dispar
isolates showed higher IC50 values when compared to E. histolytica
or reference strain. This is the first report of in vitro drug sensitivity
pattern to clinical isolates of E. histolytica and E. dispar.
There is definitely a need to monitor the random drug susceptibility among
clinical isolates especially in context to widespread use of metronidazole and
tinidazole, which are available over the counter in many countries. Increased
awareness and continued surveillance for the possible emergence of resistance
among clinical isolates is necessary for the ultimate prevention and control of
amoebiasis.
|

