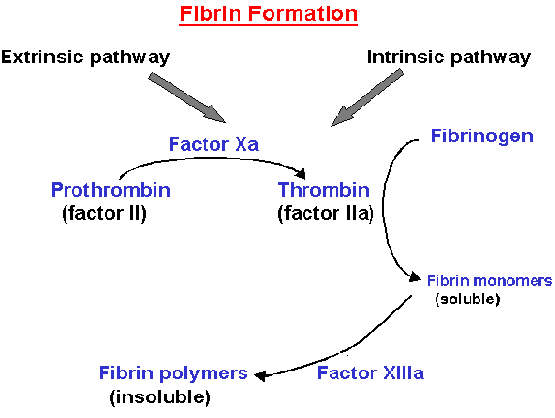
The characteristic feature of the
coagulation pathway is that upon activation the individual glycoprotein serves
as a enzyme to convert the zymogen form of the succeeding glycoprotein to its
protease form only in the presence of Ca+2 cations and on an
appropriate phospholipid membrane. The activated forms of the glycoproteins are
identified by symbol 'a'.
Both the intrinsic and the extrinsic
coagulation pathways proceed through a common pathway by forming activated
factor X. Prothrombin is cleaved at two sites by factor Xa to yied thrombin (To
see a structure of thrombin molecule click here). Ten
g-carboxyglutamic acid
residues are located on the N-terminal end of the prothrombin molecule. Vitamin
K is required in the liver biosynthesis of the prothrombin
g-carboxyglutamic groups by
participating in the carboxylation of the g-carbon of glutamic acid. These
carboxy groups are required for binding calcium to prothrombin, which induce a
conformation change in prothrombin enabling it to bindi to co-factors on the
phospholipid surfaces during its conversion to thrombin by factor Xa, factor V,
and platelet phospholipids in the presence of calcium.
Thrombin, in turn, cleaves fibrinogen.
Fibrinogen comprises 2-3% of plasma protein. Thrombin specifically cleaves the
Arg-X (X is mostly Gly) peptide bond in fibrinogen to form soluble fibrin
monomers. These monomers spontaneously aggregate to form a polymeric structure
called "soft clot". This polymer is rapidly converted to a more stable "hard
clot" by the covalent cross-linking of neighboring fibrin molecules in a
reaction catalyzed by fibrin-stabilizing factor (FSF
or XIIIa).
Types of Anticoagulants
The multilevel cascade of blood
clotting system permits enormous amplification of its triggering signals. Moving
down the extrinsic pathway, for example, proconvertin (VII), Stuart factor (X),
prothrombin, and fibrinogen are present in plasma in concentrations of <1, 8,
150, and ~4000 mg.mL-1,
respectively. Thus a small signal is very quickly amplified to bring about
effective hemostatic control.
On the other hand, clotting must be
very strictly regulated because even one inappropriate clot can have fatal
consequences. Indeed, blood clots are the leading cause of strokes and heart
attack, the two major causes of human death.
Endogenous Inhibitors of
Clotting
Thrombin plays a pivotal role in
blood coagulation and Nature has designed several serine protease
inhibitors (SERPINS) to regulate the its activity. These include
antithrombin (major), heparin cofactor II, a2-macroglobulin,
and a1-proteinase
inhibitor.
Antithrombin
is present in the plasma in significant
concentrations (~2-3 mM).
Antithrombin primarily neutralizes factor Xa and thrombin, in addition to
inhibiting most active serine proteases of the clotting system.
Protein C is another plasma
protein that limits clotting by being activated by thrombin to proteolytically
inactivate proaccelerin (V) and antihemophilic factor (VIII).
Thrombomodulin,
a cell membrane bound glycoprotein lining the vascular endothelium, specifically
binds thrombin so as to convert it to a form with decreased ability to catalyze
clot formation but with a >1,000-fold increased capacity to activate protein C.
Exogenous Inhibitors of Clotting
The control of clotting is a major
medical concern. Several inhibitors have been developed with different
mechanisms of anticoagulant action. These include the
heparins, the
coumarins, and the
1,3-indanediones.
|

