Visible and Ultraviolet Spectroscopy
1. Background
An obvious difference between certain compounds is their color. Thus, quinone
is yellow; chlorophyll is green; the 2,4-dinitrophenylhydrazone derivatives of
aldehydes and ketones range in color from bright yellow to deep red, depending
on double bond conjugation; and aspirin is colorless. In this respect the human
eye is functioning as a spectrometer analyzing the light reflected from the
surface of a solid or passing through a liquid. Although we see sunlight (or
white light) as uniform or homogeneous in color, it is actually composed of a
broad range of radiation wavelengths in the ultraviolet (UV), visible and
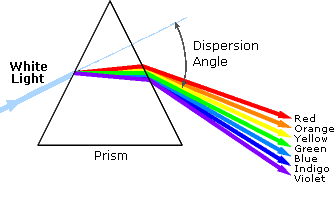
infrared (IR) portions of the spectrum. As shown on the right, the component
colors of the visible portion can be separated by passing sunlight through a
prism, which acts to bend the light in differing degrees according to
wavelength. Electromagnetic radiation such as visible light is commonly treated
as a wave phenomenon, characterized by a wavelength or frequency.
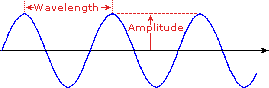
Wavelength
is defined on the left below, as the distance between adjacent peaks (or
troughs), and may be designated in meters, centimeters or nanometers (10-9
meters). Frequency is the number of wave cycles that travel past a fixed
point per unit of time, and is usually given in cycles per second, or hertz
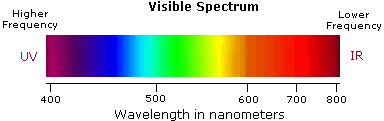
(Hz). Visible wavelengths cover a range from approximately 400 to 800 nm. The
longest visible wavelength is red and the shortest is violet. Other common
colors of the spectrum, in order of decreasing wavelength, may be remembered by
the mnemonic: ROY G BIV. The wavelengths of what we perceive as
particular colors in the visible portion of the spectrum are displayed and
listed below. In horizontal diagrams, such as the one on the bottom left,
wavelength will increase on moving from left to right.
- Violet: 400 - 420 nm
- Indigo: 420 - 440 nm
- Blue: 440 - 490 nm
- Green: 490 - 570 nm
- Yellow: 570 - 585 nm
- Orange: 585 - 620 nm
- Red: 620 - 780 nm
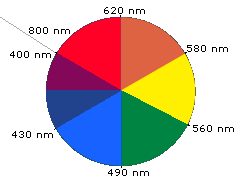
When white light passes through or is reflected by a colored substance, a
characteristic portion of the mixed wavelengths is absorbed. The remaining light
will then assume the complementary color to the wavelength(s) absorbed. This
relationship is demonstrated by the color wheel shown on the right. Here,
complementary colors are diametrically opposite each other. Thus, absorption of
420-430 nm light renders a substance yellow, and absorption of 500-520 nm light
makes it red. Green is unique in that it can be created by absoption close to
400 nm as well as absorption near 800 nm.
Early humans valued colored pigments, and used them for decorative purposes.
Many of these were inorganic minerals, but several important organic dyes were
also known. These included the crimson pigment, kermesic acid, the blue dye,
indigo, and the yellow saffron pigment, crocetin. A rare dibromo-indigo
derivative, punicin, was used to color the robes of the royal and wealthy. The
deep orange hydrocarbon carotene is widely distributed in plants, but is not
sufficiently stable to be used as permanent pigment, other than for food
coloring. A common feature of all these colored compounds, displayed below, is a
system of extensively conjugated pi-electrons.
|