4. Fragmentation Patterns
The fragmentation of molecular ions into an assortment of fragment ions is a
mixed blessing. The nature of the fragments often provides a clue to the
molecular structure, but if the molecular ion has a lifetime of less than a few
microseconds it will not survive long enough to be observed. Without a molecular
ion peak as a reference, the difficulty of interpreting a mass spectrum
increases markedly. Fortunately, most organic compounds give mass spectra that
include a molecular ion, and those that do not often respond successfully to the
use of milder ionization conditions. Among simple organic compounds, the most
stable molecular ions are those from aromatic rings, other conjugated
pi-electron systems and cycloalkanes. Alcohols, ethers and highly branched
alkanes generally show the greatest tendency toward fragmentation.
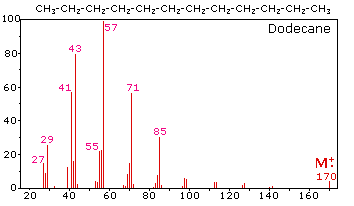
The mass spectrum of dodecane on the right illustrates the behavior of an
unbranched alkane. Since there are no heteroatoms in this molecule, there are no
non-bonding valence shell electrons. Consequently, the radical cation character
of the molecular ion (m/z = 170) is delocalized over all the covalent bonds.
Fragmentation of C-C bonds occurs because they are usually weaker than C-H
bonds, and this produces a mixture of alkyl radicals and alkyl carbocations. The
positive charge commonly resides on the smaller fragment, so we see a homologous
series of hexyl (m/z = 85), pentyl (m/z = 71), butyl (m/z = 57), propyl (m/z =
43), ethyl (m/z = 29) and methyl (m/z = 15) cations. These are accompanied by a
set of corresponding alkenyl carbocations (e.g. m/z = 55, 41 &27) formed by loss
of 2 H. All of the significant fragment ions in this spectrum are even-electron
ions. In most alkane spectra the propyl and butyl ions are the most abundant.
The presence of a functional group, particularly one having a heteroatom Y
with non-bonding valence electrons (Y = N, O, S, X etc.), can dramatically alter
the fragmentation pattern of a compound. This influence is thought to occur
because of a "localization" of the radical cation component of the molecular ion
on the heteroatom. After all, it is easier to remove (ionize) a non-bonding
electron than one that is part of a covalent bond. By localizing the reactive
moiety, certain fragmentation processes will be favored. These are summarized in
the following diagram, where the green shaded box at the top displays examples
of such "localized" molecular ions. The first two fragmentation paths lead to
even-electron ions, and the elimination (path #3) gives an odd-electron ion.
Note the use of different
curved
arrows to show single electron shifts compared with electron pair shifts.
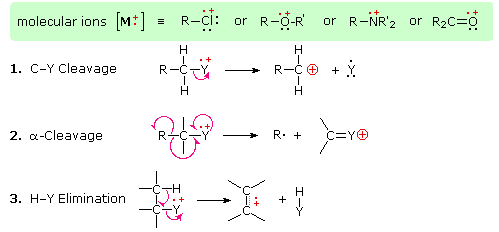
The charge distributions shown above are common, but for each cleavage
process the charge may sometimes be carried by the other (neutral) species, and
both fragment ions are observed. Of the three cleavage reactions described here,
the alpha-cleavage is generally favored for nitrogen, oxygen and sulfur
compounds. Indeed, in the previously displayed
spectra of 4-methyl-3-pentene-2-one and N,N-diethylmethylamine the major
fragment ions come from alpha-cleavages. Further examples of functional group
influence on fragmentation are provided by a selection of compounds that may be
examined by clicking the left button below. Useful tables of common fragment
ions and neutral species may be viewed by clicking the right button.
The complexity of fragmentation patterns has led to mass spectra being used
as "fingerprints" for identifying compounds. Environmental pollutants, pesticide
residues on food, and controlled substance identification are but a few examples
of this application. Extremely small samples of an unknown substance (a
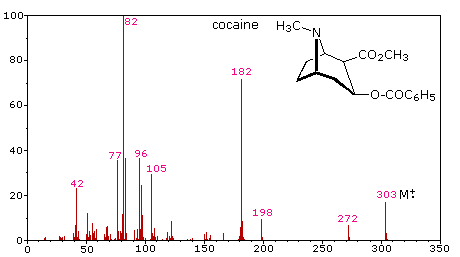
microgram or less) are sufficient for such analysis. The following mass spectrum
of cocaine demonstrates how a forensic laboratory might determine the nature of
an unknown street drug. Even though extensive fragmentation has occurred, many
of the more abundant ions (identified by magenta numbers) can be rationalized by
the three mechanisms shown above. Plausible assignments may be seen by clicking
on the spectrum, and it should be noted that all are even-electron ions. The m/z
= 42 ion might be any or all of the following: C3H6, C2H2O
or C2H4N. A precise assignment could be made from a
high-resolution m/z value (next section).
Odd-electron fragment ions are often formed by characteristic
rearrangements in which stable neutral fragments are lost. Mechanisms for some
of these rearrangements have been identified by following the course of
isotopically labeled molecular ions. A few examples of these rearrangement
mechanisms may be seen by clicking the following button.
5. High Resolution Mass Spectrometry
| Formula |
C6H12 |
C5H8O |
C4H8N2 |
| Mass |
84.0939 |
84.0575 |
84.0688 |
In assigning mass values to atoms and molecules, we have assumed integral
values for isotopic masses. However, accurate measurements show that this is not
strictly true. Because the strong nuclear forces that bind the components of an
atomic nucleus together vary, the actual mass of a given isotope deviates from
its nominal integer by a small but characteristic amount (remember E = mc2).
Thus, relative to 12C at 12.0000, the isotopic mass of 16O
is 15.9949 amu (not 16) and 14N is 14.0031 amu (not 14). By designing
mass spectrometers that can determine m/z values accurately to four decimal
places, it is possible to distinguish different formulas having the same nominal
mass. The table on the right illustrates this important feature, and a
double-focusing high-resolution mass spectrometer easily distinguishes ions
having these compositions. Mass spectrometry therefore not only provides a
specific molecular mass value, but it may also establish the molecular formula
of an unknown compound.
Tables of precise mass values for any molecule or ion are available in
libraries; however, the mass calculator provided below serves the same purpose.
Since a given nominal mass may correspond to several molecular formulas, lists
of such possibilities are especially useful when evaluating the spectrum of an
unknown compound. Composition tables are available for this purpose, and a
particularly useful program for calculating all possible combinations of H, C, N
& O that give a specific nominal mass has been written by Jef Rozenski.
|

