Nuclear Magnetic Resonance Spectroscopy
1. Background
Over the past fifty years nuclear magnetic resonance spectroscopy, commonly
referred to as nmr, has become the preeminent technique for determining the
structure of organic compounds. Of all the spectroscopic methods, it is the only
one for which a complete analysis and interpretation of the entire spectrum is
normally expected. Although larger amounts of sample are needed than for mass
spectroscopy, nmr is non-destructive, and with modern instruments good data may
be obtained from samples weighing less than a milligram. To be successful in
using nmr as an analytical tool, it is necessary to understand the physical
principles on which the methods are based.
The nuclei of many elemental isotopes have a characteristic spin (I).
Some nuclei have integral spins (e.g. I = 1, 2, 3 ....), some have fractional
spins (e.g. I = 1/2, 3/2, 5/2 ....), and a few have no spin, I = 0 (e.g. 12C,
16O, 32S, ....). Isotopes of particular interest and use
to organic chemists are 1H, 13C, 19F and
31P, all of which have I = 1/2. Since the analysis of this spin state is
fairly straightforeward, our discussion of nmr will be limited to these and
other I = 1/2 nuclei.
The following features lead to the nmr phenomenon:
1. A spinning charge generates a magnetic field, as shown by the
animation on the right.
The resulting spin-magnet has a magnetic moment (μ) proportional to the
spin.

2. In the presence of an external magnetic field (B0),
two spin states exist, +1/2 and -1/2.
The magnetic moment of the lower energy +1/2 state is alligned with the external
field, but that of the higher energy -1/2 spin state is opposed to the external
field. Note that the arrow representing the external field points North.
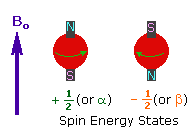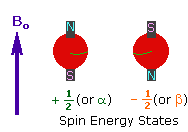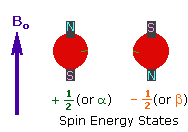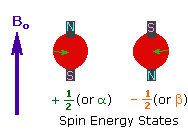
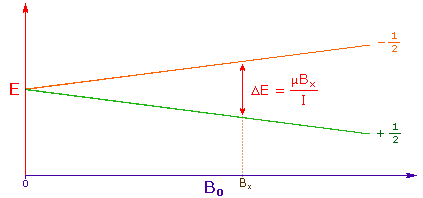
3. The difference in energy between the two spin states is dependent
on the external magnetic field strength, and is always very small. The following
diagram illustrates that the two spin states have the same energy when the
external field is zero, but diverge as the field increases. At a field equal to
Bx a formula for the energy difference is given (remember I = 1/2 and
μ is the magnetic moment of the nucleus in the field).
Strong magnetic fields are necessary for nmr spectroscopy. The international
unit for magnetic flux is the tesla (T). The earth's magnetic field is
not constant, but is approximately 10-4 T at ground level. Modern nmr
spectrometers use powerful magnets having fields of 1 to 20 T. Even with these
high fields, the energy difference between the two spin states is less than 0.1
cal/mole. To put this in perspective, recall that infrared transitions involve 1
to 10 kcal/mole and electronic transitions are nearly 100 time greater.
For nmr purposes, this small energy difference (ΔE) is usually given as a
frequency in units of MHz (106 Hz), ranging from 20 to 900 Mz,
depending on the magnetic field strength and the specific nucleus being studied.
Irradiation of a sample with radio frequency (rf) energy corresponding exactly
to the spin state separation of a specific set of nuclei will cause excitation
of those nuclei in the +1/2 state to the higher -1/2 spin state. Note that this
electromagnetic radiation falls in the
radio and television broadcast spectrum. Nmr spectroscopy is therefore the
energetically mildest probe used to examine the structure of molecules.
The nucleus of a hydrogen atom (the proton) has a magnetic moment μ = 2.7927,
and has been studied more than any other nucleus. The
previous diagram may be changed to display energy differences for the proton
spin states (as frequencies) by mouse clicking anywhere within it.
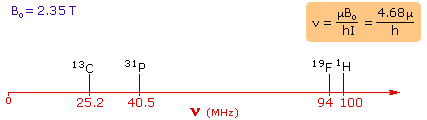
4. For spin 1/2 nuclei the energy difference between the two spin
states at a given magnetic field strength will be proportional to their magnetic
moments. For the four common nuclei noted above, the magnetic moments are:
1H μ = 2.7927, 19F μ = 2.6273, 31P μ = 1.1305 &
13C μ = 0.7022. These moments are in nuclear magnetons, which are
5.05078�10-27 JT-1. The following diagram gives the
approximate frequencies that correspond to the spin state energy separations for
each of these nuclei in an external magnetic field of 2.35 T. The formula in the
colored box shows the direct correlation of frequency (energy difference) with
magnetic moment (h = Planck's constant = 6.626069�10-34 Js).
|

