Potentiometry
Introduction
Potentiometry is the field of electroanalytical chemistry in which potential is measured under the
conditions of no current flow. The measured potential may then be used to
determine the analytical quantity of interest, generally the concentration of
some component of the analyte solution. The potential that develops in the
electrochemical cell is the result of the free energy change that
would occur if the chemical phenomena were to proceed until the equilibrium
condition has been satisfied.
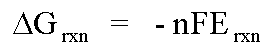
This concept is typically introduced in quantitative analysis courses in
relation to electrochemical cells that contain an anode and a
cathode. For these electrochemical cells, the potential difference
between the cathode electrode potential and the anode electrode potential
is the potential of the electrochemical cell.

If the reaction is conducted under standard state conditions, this
equation allows the calculation of the standard cell potential. When the
reaction conditions are not standard state, however, one must utilize the
Nernst equation to determine the cell potential.
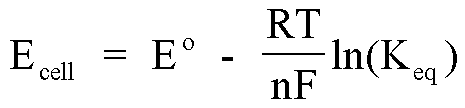
Physical phenomena which do not involve explicit redox reactions, but whose
initial conditions have a non-zero free energy, also will generate a potential.
An example of this would be ion concentration gradients across a semi-permeable
membrane. This can also be a potentiometric phenomena, and is the basis of
measurements that use ion-selective electrodes.
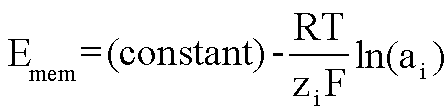
|