THE MICROCIRCULATION
CAPILLARY FILTRATION AND RESORPTION: STARLING
PRINCIPLE FOR CAPILLARY EXCHANGE:
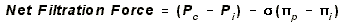
- Filtration: Blood leaving
capillary and entering organ. Net flow outward.
- Pc, capillary hydrostatic
pressure contributes to this outflow.
- PIi, interstitial oncotic
pressure contributes to this outflow. It is the oncotic
(osmotic) pressure created by insoluble proteins in the interstitial
space.
- Absorption: Blood leaving organ
and entering capillary. Net flow into capillary.
- Pi, interstitial hydrostatic
pressure, does not contribute to absorption under normal
circumstances. It is ~ 0.
- PIp, capillary oncotic
pressure, is the primary contributor to resorption.
This is the osmotic pressure created by insoluble proteins in the blood.
- sigma, REFLECTION COEFFICIENT: It
is equal to the percentage of proteins that are impermeable to the capillary
membrane, i.e. a value between 0 and 1.
- sigma = 1: Proteins are totally impermeable;
all of them are "reflected" off the membrane, thus oncotic pressure has
the greatest influence possible on net filtration.
- sigma = 0: Proteins are completely permeable,
hence no proteins are impermeable and oncotic pressures become zero.
- Low reflection coefficient affects
resorption but not so much filtration, since the capillary
oncotic pressure is the only significant force for resorption.
- RESULT: Edema.
- Lymphatics: Under normal circumstances,
filtration is greater than absorption. Thus more blood is
being deposited in organ systems than is being taken up. The difference is
put back into the blood through the lymphatic system.
- The entire blood circulation is turned around
through the lymphatic system every 24 hrs.
- Capillary Hydrostatic Pressure:
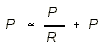
- Ra = arterial resistance
is normally much larger than venous resistance. We can usually safely
ignore venous resistance in the calculation.
- Pv = venous pressure
- Pa = mean arterial pressure
- INFLUENCES ON FILTRATION:
- ARTERIOLAR RESISTANCE: Note that
local(arteriolar) changes to vascular tone have an exact opposite effect
as systemic (large artery) changes.
- All things constant, increased
arterial resistance ------> lower capillary hydrostatic pressure
+ lower filtration
- Vasoconstriction or dilation at the
level of the arterioles does not affect MABP.
- Arteriolar Vasoconstriction ------> ------>
lower filtration rate.
- Arteriolar Vasodilation ------>------>
higher filtration rate.
- VENOUS PRESSURE: Increased Venous Pressure
------> Higher Capillary Hydrostatic Pressure ------> Increased Net
Filtration, because of the hydrostatic pressure equation:
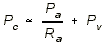
- The capillary pressure must increase in
order to achieve filtration in face of the increased venous
pressure.
- ONCOTIC PRESSURE: Negative nitrogen balance or
protein malnutrition (kwashiorkor) will lead to low
plasma albumin ------> low plasma oncotic pressure ------> low or no
resorption, which means high net rate of filtration ------>
edema, ascites
CAPILLARY PERMEABILITY:
- Three types of capillaries, each having different
levels of permeability:
- Continuous: Tight Junctions, as in brain,
thymus, retina.
- Fenestrated: Little diaphragms where diffusion
can take place, having somewhat higher permeability. GI-Tract.
- Discontinuous: Liver sinusoids, complete
discontinuities in the system.
- Endothelial Cells: When
endothelial cells contract, the spaces between them increase ------> higher
capillary permeability.
- Mast Cell Degranulation leads
to release of Histamine and Platelet-Activating
Factor (PAF).
- This makes the endothelial cell release
Calcium from the SR ------> actin-myosin contraction
of endothelial cell makes the cell change shape ------> more
spaces between the cells.
- Anaphylactic Shock: High capillary permeability
leads to low blood pressure. We can't just give them fluids to increase
blood volume, because the fluids leak right out again.
EDEMA: It can occur from a lot of sources, such as no
resorption. Consequences of edema:
- Impair Exchange of Metabolites: It leads to bigger
spaces (longer distance) between capillaries and the tissues ------>
diffusion becomes impossible.
- THE EDEMA POSITIVE-FEEDBACK CYCLE: Edema can
compress venules ------> higher venous return ------> higher CVP ------>
higher hydrostatic pressure and filtration rate ------> even more edema.
LYMPHATIC BLOCKAGE: If you block lymphatics, then
interstitial fluid along with interstitial proteins will rise ------>
increase interstitial oncotic pressure ------> more filtration ------> massive
edema.
VASCULAR SMOOTH MUSCLE: Anything that increases
intracellular Ca+2 concentration will increase contractility of
vascular muscle.
- VASCULAR CONTRACTION:
- Mechanism of Contraction, briefly:
- Calcium binds to Calmodulin
- The Ca+2-Calmodulin Complex then
binds to Myosin Light-Chain Kinase
- This results in myosin being free to
interact with actin.
- Vascular Tone: The overall
rate of cross bridging is much slower than in vascular smooth muscle.
There is always a baseline level of activity = vascular tone.
- Norepinephrine will cause
vascular contraction by increasing Ca+2 in
smooth muscle, via three pathways:
- NorE can directly open Ca+2-Channels
- NorE can bind to alpha1-Receptors
to active the alpha-Adrenergic Pathway (DAG/IP3) ------>
higher intracellular Ca+2
- Voltage-Gated Ca+2 Channels can
further open, in response to the above two.
- VASCULAR RELAXATION: Anything that decreases Ca+2
concentration will cause relaxation.
- Epinephrine in the blood
causes vascular relaxation.
- Epi binds beta2-Receptors
to activate beta-Adrenergic Pathway ------> higher levels of cAMP
which results in decreased Ca+2 in cytosol.
- cAMP will facilitate pumping of Ca+2
back into SR.
- ATP: Low levels of ATP will
cause vascular relaxation locally, which should allow
greater blood flow, greater perfusion, and hence more ATP to deprived
tissue.
- ATP-Dependent K+-Channels
open in response to LOW ATP.
- This leads to Hyperpolarization ------>
Vascular Relaxation ------> greater blood flow to area.
- NO causes relaxation, covered
later.
- VASOMOTION: Spontaneous action
potentials can cause a cyclic change in vascular tone.
- Addition of NorEpi increases
the rate of firing of those action potentials ------> more vascular
tone.
- However, action potentials are not always
required to cause sustained contraction.
ENDOTHELIAL-DERIVED FACTORS: Nitric Oxide
- EXPT: Acetylcholine's (i.e. parasympathetic) effect
on vessels depends on the presence of the endothelial cells.
- Add Ach to vessel with endothelial cells intact
------> relaxation.
- Add Ach to vessel with endothelial cells
removed ------> actually leads to contraction!
- Process of NO-Mediated Vascular Relaxation:
- Ach binds to endothelial cell.
- Ca+2 channels open and Ca+2
pours into endothelium.
- This makes the endothelial cell produce NO from
Arginine, by up-regulating synthesis of the enzyme Constitutive
NO-Synthase.
- Endothelium makes NO which diffuses to the
underlying vascular smooth muscle.
- NO then activates Guanylyl Cyclase, which
produces cGMP ------> leads to Ca+2
sequestration and vascular relaxation.
- L-Nitroarginine Methyl Ester (L-NAME):
Inhibits NO-Synthase, blocking production of NO ------> arteriolar
constriction ------> lower blood flow to region.
- ISCHEMIA-REPERFUSION: The danger in reperfusing
ischemic tissue is that massive influx of O2 can lead to
oxidative free radicals which damage endothelial
cells. The free radicals have two bad effects:
- They react with NO, leading to vasoconstriction
and reduced perfusion of the area.
- They directly damage the endothelial membrane
leading to increased vascular permeability which isn't good (it
can lower blood pressure, etc.)
- SEPSIS: Causes vesicle to become
less sensitive to vasoconstriction. Phenylephrine has a lesser
effect on septic vessels.
- It leads to higher NO via Inducible
NO-Synthase. This is not the same enzyme as
constitutive NO-Synthase.
- The number of vasoconstrictive alpha-Receptors
is decreased.
- Basal Ca+2 levels are reduced or Ca+2-channels
don't open properly.
- ADHESION MOLECULES: NO
protectively prevents expression of adhesion molecules, so that leucocytes
don't stick to vessel wall, which can lead to microvascular injury.
- Hence we can't use L-NAME as a treatment for
Sepsis -- we need the NO to prevent sticking of blood cells, even if
vasodilation is an undesired effect.
- What we need is a drug that blocks only
Inducible NO-Synthase (made during sepsis) and not constitutive
NO-Synthase. We don't have that (yet).
ENDOTHELIN-1: Vasoconstrictive agent
produced by endothelial cells.
- SLOW-RESPONSE: Endothelin is not stored in
vesicles. It is synthesized de novo. Thus it is a slow (long-term)
response.
- SYNTHESES: Preproendothelin ------> Big
Endothelin ------> Endothelin. Multi step synthesis adds to slow
response.
- EFFECT: Endothelin causes sustained
vasoconstriction. The effect lasts long! It causes increased levels of
Ca+2 and thus increased vascular tone.
- It acts via alpha-adrenergic pathway (PIP/DAG
------> Ca+2)
- It also acts directly on Ca+2-Channels.
- ISCHEMIA REPERFUSION: Endothelin is bad! It
can be released along with inflammatory mediators to cause further
vasoconstriction when we want vasodilation.
LOCAL REGULATION OF BLOOD FLOW:
-
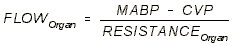
- We control local blood flow by changing local
resistance.
- Three factors can change local resistance:
- Endothelial-Derived Factors
- Mechanical Stretch of the vessel itself
- Intrinsic Factors = locally
derived metabolites
- Organ-Distribution of Blood Flow: Highest
perfusion rates are in liver, kidney, and skeletal muscle.
- Kidneys have the highest
Perfusion Index: The ratio of perfusion to organ size.
It measures the relative amount of blood that different organs get per
organ mass.
- OXYGEN UPTAKE:
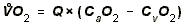
- To increase Oxygen Uptake by tissues, you can
therefore increase one of two things. Most organs increase O2-uptake
by a combo of both things.
- Increase O2 extraction.
This is how the KIDNEYS primarily get more oxygen.
- Increase blood flow. This is how
the HEART primarily gets more oxygen. The heart
can't increase O2-extraction because it is already
extracting about the maximum amount possible.
- O2-Extraction = Arterial PO2
- Venous PO2
- Oxygen is extracted by simple diffusion.
- To increase oxygen extraction, increase the
surface area of capillaries exposed to tissue.
- Heart-Muscle has a high basal capillary
concentration than skeletal muscle. Thus it has higher oxygen
extraction.
- Pre-Capillary Sphincters
can be dilated to perfuse more capillaries in the
capillary bed.
- Specific Organs:
- HEART: It has a high oxygen extraction,
so the only way to increase O2 uptake is to
increase blood flow.
- KIDNEY: It has a lower oxygen extraction.
It can actually increase O2 extraction
to increase O2-Uptake.
AUTOREGULATION:
- Mechanism: Keep constant flow and capillary
pressure (i.e. filtration) in the face of changing systemic pressures.
- Lower local pressure ------> Vasodilate
------> lower resistance ------> maintain higher flow and higher
capillary pressure.
- Higher local pressure ------>
Vasoconstrict ------> higher resistance ------> maintain lower
flow and lower capillary pressure
- Tissues: Autoregulation works particularly in the
kidney, heart, and brain.
- Limits: Autoregulation only works in a limited
range of pressures. Vessels won't change diameter past their minimum and
maximum.
MYOGENIC RESPONSE: Sudden stretch of
vascular wall can lead to vasoconstriction to counteract the higher
blood-volume. Works in conjunction with the metabolic response to maintain blood
flow.
- There are two types of arterioles:
- One produces Action Potentials to have rhythmic
vasoconstriction (vasomotion)
- The other type does not produce action
potentials.
- Both types are still subject to the myogenic
response.
- Mechanism:
- AP-Capable Arterioles: Stretch ------>
increased frequency of AP-firing ------> higher vascular tone.
- AP-Incapable Arterioles: Stretch
------> depolarization of vascular smooth muscle ------> Ca+2
influx and higher vascular tone.
METABOLIC RESPONSES: Works in
conjunction with the Myogenic Response to maintain blood flow.
- METABOLIC HYPOTHESIS: Vasodilator Metabolites are
made locally in response to hypoxia and poor blood flow, in order to
increase blood flow. The metabolites are then washed away when blood flow
increases again, disposing of their effect.
- HYPOXIA: Hypoxia leads to a decrease in
intracellular ATP, which ultimately leads to vasodilation.
- K+-ATP CHANNELS:
They kick K+ out of the cell in exchange for bringing ATP in.
They open in response to low ATP levels.
- Hypoxia ------> low intracellular ATP ------>
Open K+-ATP Channels ------> K+ pours out of cell
------> membrane hyperpolarizes ------> smooth muscle
relaxation.
- PROSTACYCLIN (PGI2):
Prostacyclin may be released by endothelial cells in response to hypoxia
------> potent vasodilation in a paracrine manner no neighboring smooth
muscle.
- ACIDOSIS: Acidosis in smooth muscle directly causes
hyperpolarization of smooth muscle membrane ------> vasodilation.
- Acidosis means CO2 levels in tissue
are high.
- CO2 <====> H2CO3
<====> H+ + HCO3-
- ADENOSINE: Adenosine is an indicator that the
target tissue is out of ATP (as opposed to the smooth muscle itself).
- Adenosine is membrane-soluble while ATP, ADP,
and AMP are not. So when the compounds gets down to the Adenosine level,
it can then leave the cell to affect the neighboring smooth muscle.
- Adenosine is a potent vasodilator.
- AUTOCOIDS: Histamine, Bradykinin, Serotonin,
Prostaglandins, Leukotrienes.
- POTASSIUM: Potassium regulation is
especially important in the brain and in skeletal muscle.
- SMALL AMOUNTS OF K+
- In both tissues, extracellular K+
concentration goes up because of repeated firing of action
potentials.
- This results in release of
vasodilator-factors (NO, PGI2) and in membrane
hyperpolarization of vascular smooth muscle.
- HUGE (PHARMACOLOGICAL) INCREASE IN K+
------> depolarization of muscle membrane ------> vasoconstriction.
- INTERSTITIAL OSMOLARITY:
ACTIVE HYPEREMIA: Blood flow changes in
proportion to changes in metabolic activity of the organ. Occurs in Skeletal
Muscle.
- Lactic Acidosis in skeletal muscle ------>
Vasodilation of vasculature.
- In Active Hyperemia, the metabolic activity of the
target tissue (i.e. skeletal muscle) is changing, and that's what
causing the vasodilation.
REACTIVE HYPEREMIA: The short-term
increase in flow following temporary ischemia to a region.
- Both myogenic and metabolic effects are playing a
role in causing the vasodilation.
- In reactive hyperemia, the metabolic activity of
the target tissue does not change, whereas in active hyperemia, it does.
REGIONAL CIRCULATIONS:
- CEREBRAL CIRCULATION:
- Cerebrospinal Fluid: Normally has a lower
protein content than blood.
- Cerebral Vasculatures have very poor
sympathetic innervation. Hence in the Cushing Reflex,
massive sympathetics don't cause constriction of vessels in the cerebrum
(which they shouldn't!)
- Regulation of Flow: It is primarily K+-Mediated.
We can get higher extracellular K+ and vascular
hyperpolarization by two sources:
- Firing of neurons without repolarization.
- K+-ATPase kicks out K+
in exchange for ATP, at low intracellular ATP levels.
- The brain is very sensitive to changes in
PCO2, but not so much to changes in PO2.
- CORONARY CIRCULATION: Regulated almost entirely by
local factors.
- Increase cardiac work ------> increased
coronary blood flow.
- SYSTOLE: Coronary blood flow decreases, as the
vessels are squeezed as the myocardium contracts.
- There may even be some retrograde flow of
blood during systole.
- DIASTOLE: Coronary blood flow increases.
|

