Cryatallization
Crystals are grown in many shapes, which are dependent upon
downstream processing or final product requirements. Crystal shapes can include
cubic, tetragonal, orthorhombic, hexagonal, monoclinic, triclinic, and trigonal.
In order for crystallization to take place a solution must be "supersaturated".
Supersaturation refers to a state in which the liquid (solvent) contains more
dissolved solids (solute) than can ordinarily be accomodated at that
temperature.
As with any separation method, equilibrium plays an
important role. Below is a general solubility curve for a solid that forms
hydrate (a compound that has one or more water molecules attached) as it cools.
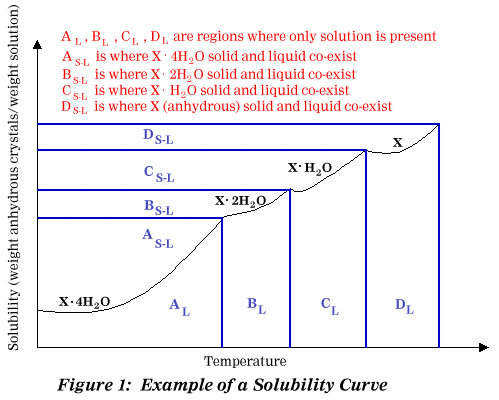
In Figure 1, X may be any solid that can form hydrates such
as Na2S2O3. The number of hydrate molecules
shown in Figure 1 is strictly arbitrary and will vary for each substance.
So how do you grow crystals? Let's consider an example
that is fairly easy to envision. Take a pot of boiling water and add table salt
while stirring to make a water-salt solution. Continue adding salt until no
more salt will dissolve in the solution (this is a saturated solution). Now add
one final teaspoon of salt. The salt that will not dissolve will help the first
step in crystallization begin. This first step is called "nucleation" or
primary nucleation. The salt resting at the bottom of the pot will provide a
site for nucleation to occur.
Primary nucleation is the first step in
crystallization. Simply defined, it's the growth of a new crystalA
On an industrial scale, a large supersaturation driving
force is necessary to initiate primary nucleation. The initiation of primary
nucleation via this driving force is not fully understood which makes it
difficult to model (experiments are the best guide). Usually, the instantaneous
formation of many nuclei can be observed "crashing out" of the solution. You
can think of the supersaturation driving force as being created by a combination
of high solute concentration and rapid cooling. In the salt example, cooling
will be gradual so we need to provide a "seed" for the crystals to grow on. In
continuous crystallization, once primary nucleation has begun, the crystal size
distribution begins to take shape. Think about our salty water, as you look at
Figure 2 describing the progression of crystallization.
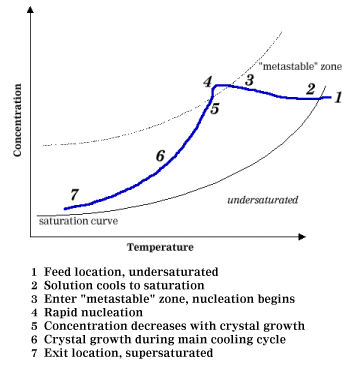
Figure 2: Progression of Crystallization
The second chief mechanism in crystallization is called
secondary nucleation. In this phase of crystallization, crystal growth is
initiated with contact. The contact can be between the solution and other
crystals, a mixer blade, a pipe, a vessel wall, etc. This phase of
crystallization occurs at lower supersaturation (than primary nucleation) where
crystal growth is optimal.
Secondary nucleation requires "seeds" or
existing crystals to perpetuate crystal growth. In our salt example, we
bypassed primary nucleation by "seeding" the solution with a final
teaspoon of salt. Secondary nucleation can be thought of as the
workhorse of crystallization.
Again, no complete theory is available to model secondary nucleation and
it's behavior can only be anticipated by experimentation. Mathematic
relationships do exist to correlate experimental data. However, correlating
experimental data to model crystallization is time consuming and often
considered extreme for batch operations, but can easily be justified for
continuous processes where larger capital expenditures are necessary. For batch
operations, only preliminary data measurements are truly necessary.
We've discussed how crystallization occurs once supersaturation is
reached, but how do we reach supersaturation? We have already covered one such
method in our salt crystallization example. Since the solubility of salt in
water decreases with decreasing temperature, as the solution cools, its
saturation increases until it reaches supersaturation and crystallization begins
(Figure 3). Cooling is one of the four most common methods of achieving
supersaturation. It should be noted that cooling will only help reach
supersaturation in systems where solubility and temperature are directly
related. Although this is nearly always the case, there are exceptions. In
Figure 3, you'll note that Ce2(SO4)3 actually
becomes less soluble in water at higher temperatures.
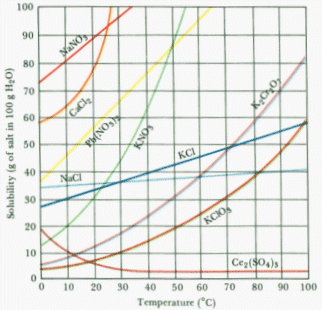
Figure 3: Solubilities of Several Solids
The four most common methods of reaching supersaturation in
industrial processes are:
1. Cooling (with some exceptions)
2. Solvent Evaporation
3. Drowning
4. Chemical Reaction
In an industrial setting, the
solute-solvent mixture is commonly referred to as the "mother
liquor". Drowning describes the addition of a nonsolvent to the
solution which decreases the solubility of the solid. A chemical reaction can
be used to alter the dissolved solid to decrease its solubility in the solvent,
thus working toward supersaturation. Each method of achieving supersaturation
has its own benefits. For cooling and evaporative crystallization,
supersaturation can be generated near a heat transfer surface and usually at
moderate rates. Drowning or reactive crystallization allows for localized,
rapid crystallization where the mixing mechanism can exert significant influence
on the product characteristics.
Equipment Used in Crystallization
1.Tank Crystallizers
This is probably the oldest and most basic method of
crystallization. In fact, the "pot of salt water" is a good example of tank
crystallization. Hot, saturated solutions are allowed to cool
in open tanks. After crystallization, the mother liquor is drained and the
crystals are collected. Controlling nucleation and the size of the crystals is
difficult. The crystallization is essentially just "allowed to happen". Heat
transfer coils and agitation can be used. Labor costs are high, thus this type
of crystallization is typically used only in the fine chemical or pharmaceutical
industries where the product value and preservation can justify the high
operating costs.
2.Scraped Surface Crystallizers
An example may be the Swenson-Walker crystallizer consisting of a
trough about 2 feet wide with a semi-circular bottom. The outside is jacketed
with cooling coils and an agitator blade gently passes close to the trough wall
removing crystals that grow on the vessel wall.
3.Forced Circulating Liquid Evaporator-Crystallizer
Just as the name implies, these crystallizers combine
crystallization and evaporation, thus the driving forces toward supersaturation.
The circulating liquid is forced through the tubeside of a steam heater. The
heated liquid flows into the vapor space of the crystallization vessel. Here,
flash evaporation occurs, reducing the amount of solvent in the solution
(increasing solute concentration), thus driving the mother liquor towards
supersaturation. The supersaturated liquor flows down through a tube, then up
through a fluidized area of crystals and liquor where crystallization takes
place via secondary nucleation. Larger product crystals are withdrawn while the
liquor is recycled, mixed with the feed, and reheated.
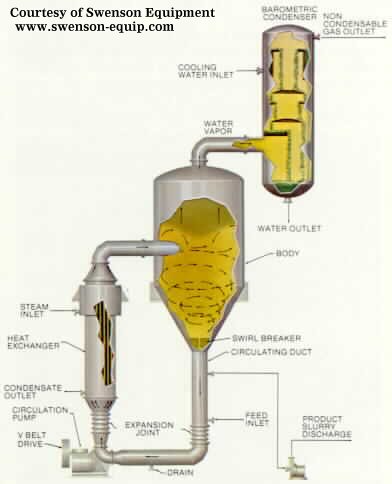
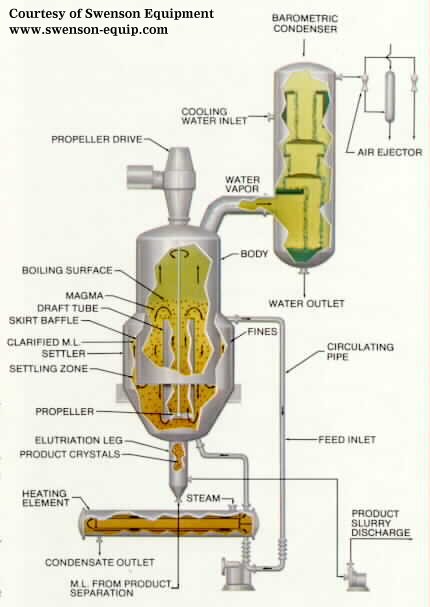
4.Circulating Magma Vacuum Crystallizer
In this type of crystallizer, the crystal/solution mixture (magma)
is circulated out of the vessel body. The magma is heated gently and mixed back
into the vessel. A vacuum in the vapor space causes boiling at the surface of
the liquid. The evaporation causes crystallization and the crystals are drawn
off near the bottom of the vessel body.
|

