Glass Transition
Have you ever left a plastic bucket or some other plastic object outside
during the winter, and found that it cracks or breaks more easily than it would
in the summer time? What you experienced was the phenomenon known as the
glass transition. This transition is something that only happens to
polymers, and is one of the things that make polymers unique. The glass
transition is pretty much what it sounds like. There is a certain
temperature(different for each polymer) called the glass transition
temperature, or Tg for short. When the polymer is cooled below this
temperature, it becomes hard and brittle, like glass. Some polymers are used
above their glass transition temperatures, and some are used below. Hard
plastics like polystyrene and
poly(methyl methacrylate), are used
below their glass transition temperatures; that is in their glassy state
Their Tg's are well above room temperature, both at around 100 oC.
Rubber elastomers like
polyisoprene and
polyisobutylene, are used above
their Tg's, that is, in the rubbery state, where they are soft and
flexible.
Amorphous and Crystalline Polymers
We have to make something clear at this point. The glass transition is not
the same thing as melting. Melting is a transition which occurs in
crystalline polymers. Melting
happens when the polymer chains fall out of their crystal structures, and become
a disordered liquid. The glass transition is a transition which happens to
amorphous polymers; that is, polymers whose chains are not arranged in
ordered crystals, but are just strewn around in any old fashion, even though
they are in the solid state.
But even crystalline polymers will have a some amorphous portion. This
portion usually makes up 40-70% of the polymer sample. This is why the same
sample of a polymer can have both a glass transition temperature and a
melting temperature. But you should know that the amorphous portion undergoes
the glass transition only, and the crystalline portion undergoes melting
only.
The Snake Pit
Now, to understand just why polymers with no order to them are hard and
brittle below a certain temperature and soft and pliable above it, it can help
to think of a polymer in the amorphous state as a big room full of slithering
snakes. Each snake is a polymer chain. Now as you may remember, snakes are cold
blooded animals, so all their body heat has to come from their surroundings.
When it's warm, the snakes are happy, and can go on about their business of
slithering and sliding with no trouble at all. They will move all about
randomly, over and around each other, and they slither hither and thither, just
having a great time, or as good a time as snakes ever have.
But when it gets cold, snakes don't move too much. They slow down without
any heat, and tend to just sit still. Now they're still all wrapped around,
over, and under each other, but as far as motion is concerned, it just doesn't
happen.
Now imagine trying to drive a bulldozer through this room full of snakes.
If it's warm, and the snakes are moving, they can quickly slither out of your
way, and the bulldozer moves through the room, causing a minimal amount of snake
damage. But if it's cold, one of two things will happen to the motionless
snakes. Either (A) the snakes will be stronger than the bulldozer, and the
bulldozer won't get through, and the snakes will stay put; or (B) the bulldozer
will be stronger than the snakes, and they'll get squashed, still not moving
anywhere.
Polymers are the same way. When the temperature is warm, the polymer
chains can move around easily. So, when you take a piece of the polymer and bend
it, the molecules, being in motion already, have no trouble moving into new
positions to relieve the stress you have placed on them. But if you try to bend
sample of a polymer below its Tg, the polymer chains won't be able to
move into new positions to relieve the stress which you have placed on them. So
just like in the example of a room full of cold snakes, one of two things will
happen. Either (A) the chains are strong enough to resist the force you apply,
and the sample won't bend; or (B) the force you apply will be too much for the
motionless polymer chains to resist, and being unable to move around to relieve
the stress, the polymer sample will break or shatter in your hands.
This change in mobility with temperature happens because the phenomenon we
call "heat" is really a form of kinetic energy; that is, the energy of objects
in motion. It is actually an effect of random motion of molecules, whether they
are polymer molecules or small molecules. Things are "hot" when their molecules
have lots of kinetic energy and move around very fast. Things are "cold" when
their molecules lack kinetic energy and move around slowly, or not at all.
Now the exact temperature at which the polymer chains undergo this big
change in mobility depends on the structure of the polymer. To see how a small
change in structure can mean a big change in Tg, take a look at the
difference between poly(methyl acrylate) and poly(methyl methacrylate) on the
acrylate page.
Twistin' the Night Away
There is a difference between polymers and snakes that we probably should
discuss at this point. An individual snake is not only wiggling around, but
actually moving from one side of the room to the other. This is called
translational motion. When you walk down the street, presuming you're not
like most Americans who never walk anywhere, you are undergoing translational
motion. While polymers are not incapable of such motion, mostly they are not
undergoing translational motion. But they are still moving around, wiggling this
way and that, much like little kids in church. To be sure, by the time we get
down to the glass transition temperature, it is already too cold for the polymer
molecules, tangled up in each other as they are, to move any distance in one
direction. The motion that allows a polymer above its glass transition
temperature to be pliable is not usually translational motion, but what is known
in the business as long-range segmental motion. While the polymer chain
as a whole may not be going anywhere, segments of the chain can wiggle around,
swing to and fro, and turn like a giant corkscrew. The polymer samples may be
thought of as a crowd of people on a dance floor. While each whole body tends to
stay in the same spot, various arms, legs, and whatnot are changing position a
lot. When the temperature drops below the Tg, for polymers the party's
over, and the long-range segmental motion grinds to a halt. When this long-range
motion ceases, the glass transition occurs, and the polymer changes from being
soft and pliable to being hard and brittle.
See for yourself
Now to make sure this is all clear, we made a little movie showing what
happens to the polymer chains at the glass transition temperature.
Try This!
Want to have some fun? First, get your teacher to bring some liquid
nitrogen to class. Then put some in a
styrofoam cup, and drop in some
household objects made from polymers, like rubber bands or plastic wrap. The
liquid nitrogen, being nippy as it is, will cool the objects below their glass
transition temperatures. Try to bend your rubber band (hold it with a pair of
pliers, because you could get frostbite if you try to touch it with your
fingers) and it will shatter! Neato, huh? The rubber band will shatter because
it's below its glass transition temperature.
Measuring the Tg
If you want to know how we measure both melting points and Tg's, plus
latent heats of melting, and changes in heat capacity, now there's a wonderful
page to tell you all about a technique called
differential scanning calorimetry.
Go visit it!
Where Next?
Want to know more about the wonderful glass transition? Read these little
segments!
- Messing Around with
the Tg
- The Tg vs. Melting
- What Becomes the High Tg
Polymer?
Messing Around with the Glass Transition
Sometimes, a polymer has a Tg that is higher
than we'd like. That's ok, we just put something in it called a plasticizer.
This is a small molecule which will get in between the polymer chains, and space
them out from each other. We call this increasing the free volume. When
this happens they can slide past each other more easily. When they slide past
each other more easily, they can move around at lower temperatures than they
would without the plasticizer. In this way, the Tg of a polymer can be
lowered, to make a polymer more pliable, and easier to work with.
If you're wondering what kind of small molecule
we're talking about, here are some that are used as plasticizers:
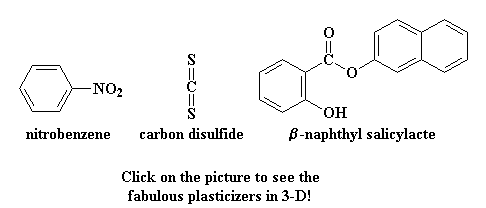
Have you ever smelled "that new car smell"? It's not something I smell too
often on the money I make, but that smell is the plasticizer evaporating from
the plastic parts on the inside of your car. After many years, if enough of it
evaporates, your dashboard will no longer be plasticized. The Tg of the
polymers in your dashboard will rise above room temperature, and the dashboard
will become brittle and crack.
The Glass Transition vs. Melting
Keywords:
first order transition,
heat capacity,
second order transition
It's tempting to think of the glass transition as a kind of
melting of the polymer. But this is an inaccurate way of looking at things.
There are a lot of important differences between the glass transition and
melting. Like I said earlier, melting is something that happens to a crystalline
polymer, while the glass transition happens only to polymers in the amorphous
state. A given polymer will often have both amorphous and crystalline domains
within it, so the same sample can often show a melting point and a Tg.
But the chains that melt are not the chains that undergo the glass transition.
There is another big difference between melting and the glass transition.
When you heat a crystalline polymer at a constant rate, the temperature will
increase at a constant rate. The heat amount of heat required to raise the
temperature of one gram of the polymer one degree Celsius is called the heat
capacity.
Now the temperature will continue to increase until the polymer reaches
its melting point. When this happens, the temperature will hold steady for
awhile, even though you're adding heat to the polymer. It will hold steady until
the polymer has completely melted. Then the temperature of the polymer will
begin to increase once again. The temperature rising stops because melting
requires energy. All the energy you add to a crystalline polymer at its melting
point goes into melting, and none of it goes into raising the temperature. This
heat is called the latent heat of melting. (The word latent means
hidden.)
Now once the polymer has melted, the temperature begins to rise again, but
now it rises at a slower rate. The molten polymer has a higher heat capacity
than the solid crystalline polymer, so it can absorb more heat with a smaller
increase in temperature.
So, two things happen when a crystalline polymer melts: It absorbs a
certain amount of heat, the latent heat of melting, and it undergoes a change in
its heat capacity. Any change brought about by heat, whether it is melting or
freezing, or boiling or condensation, which has a change in heat capacity, and a
latent heat involved, is called a first order transition.
But when you heat an amorphous polymer to its Tg, something
different happens. First you heat it, and the temperature goes up. It goes up at
a rate determined by the polymer's heat capacity, just like before. Only
something funny happens when you reach the Tg. The temperature doesn't
stop rising. There is no latent heat of glass transition. The temperature keeps
going up.
But the temperature doesn't go up at the same rate above the Tg as
below it. The polymer does undergo an increase in its heat capacity when it
undergoes the glass transition. Because the glass transition involves change in
heat capacity, but it doesn't involve a latent heat, this transition is called a
second order transition.
It may help to look at some nifty pictures. The plots show the amount of
heat added to the polymer on the y-axis and the temperature that you'd
get with a given amount of heat on the x-axis.

The plot on the left shows what happens when you heat a 100% crystalline
polymer. You can look at it and see that it's discontinuous. See that break?
That's the melting temperature. At that break, a lot of heat is added without
any temperature increase at all. That's the latent heat of melting. We see the
slope getting steeper on the high side of the break. The slope of this kind of
plot is equal to the heat capacity, so this increase in steepness corresponds to
our increase in heat capacity above the melting point.
But in the plot on the right, which shows what happens to a 100% amorphous
polymer when you heat it, we don't have a break. The only change we see at the
glass transition temperature is an increase in the slope, which means, of
course, that we have an increase in heat capacity. We can see a heat capacity
change at the Tg, but no break, like we do in the plot for the
crystalline polymer. As I said before, there is no latent heat involved with the
glass transition.
And this, my friends, right before your eyes, is the difference between a
first order transition like melting, and a second order transition like the
glass transition.
What Becomes the High Tg Polymer?
Ok, we know at this point that some polymers have high Tg's,
and some have low Tg's. The question we haven't bothered to ask yet is
this: why? What makes one polymer glass transition at 100 oC
and another at 500 oC?
The very simple answer is this: How easily the chains move.
A polymer chain that can move around fairly easily will have a very low Tg,
while one that doesn't move so well will have a high one. This makes sense. The
more easily a polymer can move, the less heat it takes for the chains to
commence wiggling and break out of the rigid glassy state and into the soft
rubbery state.
So then I suppose we've brought ourselves to another
question...
What makes one polymer move more easily than
another?
I'm glad you asked that. There are several things that affect
the mobility of a polymer chain. Go look at each one!
- Backbone Flexibility
- Pendant Groups Part I:
Fish Hooks and Boat Anchors
- Pendant Groups Part II:
Elbow Room
Backbone Flexibility
This is the biggest and most important one to remember. The
more flexible the backbone chain is, the better the polymer will move, and the
lower its Tg will be. Let's look at some examples. The most dramatic one
is that of silicones. Let's
take a look at one called polydimethylsiloxane.
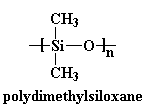
This backbone is so flexible that polydimethylsiloxane has a Tg way
down at -127 oC! This chain is so flexible that it's a liquid at room
temperature, and it's even used to thicken shampoos and conditioners.
Now we'll look at another extreme, poly(phenylene sulfone).
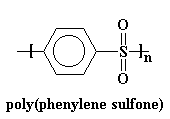
This polymer's backbone is just plain stiff. It's so rigid that it doesn't
have a Tg! You can heat this thing to over 500 oC and it will
still stay in the glassy state. It will decompose from all the heat before it
lets itself undergo a glass transition! In order to make a polymer that's at all
processable we have to put some flexible groups in the backbone chain. Ether
groups work nicely.
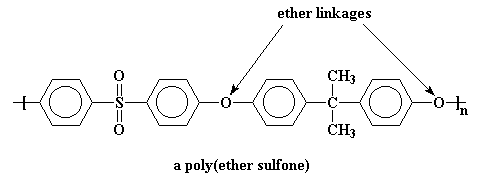
Polymers like this are called
poly(ether sulfones), and those flexible ether groups bring the Tg of
this one down to a more manageable 190 oC.
Pendant Groups Part I:
Fish Hooks and Boat Anchors
Pendant groups have a big effect on chain mobility. Even a
small pendant group can act as a fish hook that will catch on any nearby
molecule when the polymer chain tries to move like corkscrew. Pendant groups
also catch on each other when chains try to slither past each other.
One of the best pendant groups for getting a high Tg
is the big bulky adamantyl group. An adamantyl group is derived from a compound
called adamantane.
Click on the adamantane to see it in 3-D!
A big group like this does more than just act like a hook that catches on
nearby molecules and keeps the polymer from moving. It's a downright boat
anchor. Not only does it get caught on nearby polymer chains, its sheer mass is
such a load for its polymer chain to move that it makes the polymer chain move
much more slowly. To see how much this affects the Tg, just take a look
at two poly(ether ketones), one with
an adamantane pendant group and one without.
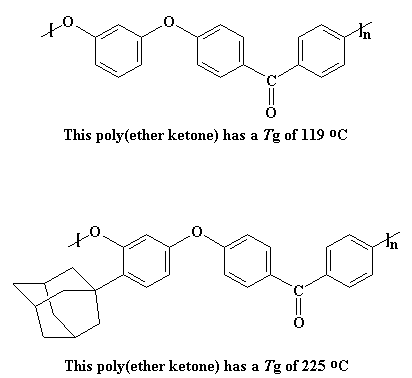
The Tg of the polymer on the top is already decent at 119 oC,
but the adamantyl group raises even higher, to 225 oC.
Pendant Groups Part II:
Elbow Room
But big bulky pendant groups can lower the Tg, too.
You see, the big pendant groups limit how closely the polymer chains can pack
together. The further they are from each other, the more easily they can move
around. This lowers the Tg, in the same way a
plasticizer does. The
fancy way to say that there is more room between the polymer chains is to say
there is more free volume in the polymer. The more free volume, the lower
the Tg generally. We can see this with a series of
methacrylate polymers:
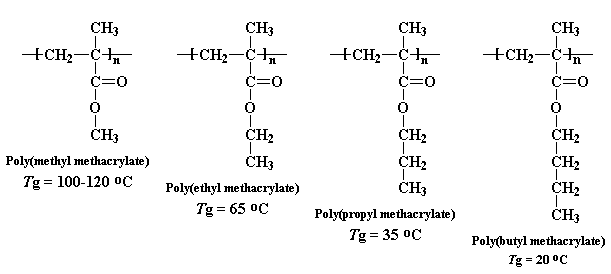
You can see a big drop each time we make that pendant alkyl chain one
carbon longer. We start out at 120 oC for
poly(methyl methacrylate), but by
the time we get to poly(butyl methacrylate) the Tg has dropped to only 20oC,
pretty close to room temperature.
|

