Molecular Architecture - Hybrid Orbitals
Please be patient, the loading of the hydrid
orbitals may take a few minutes as they are large files.
The mixing of orbitals of different energy levels gives rise to
new orbital types of intermediate energy that are able to form stronger
bonds.
The approach that we have taken so far has worked well with some
simple molecules. their shapes are explained very nicely by the overlap
of simple atomic orbitals. It does note take long to find molecules with
shapes and bond angles that fail to fir the model that has been
developed. For example, CH4, has a shape that the VSEPR
theory predicts to be tetrahedral. The H-C-H bond angles in this
molecule are 109.5o. No simple atomic orbitals are orientated
at this bond angle. So what kinds of orbitals are CH4
molecules using?
When some atoms form bonds, their simple s, p, and d orbitals
often mix to form new atomic orbitals, called hybrid atomic
orbitals. These new orbitals have new shapes and new directional
properties. The reason for this mixing can be seen if we look at their
shapes.
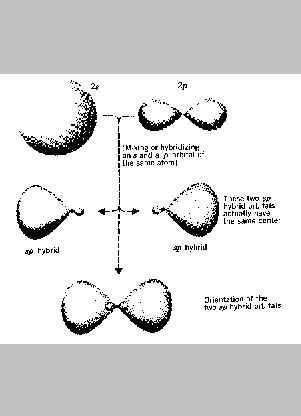
One kind of hybrid atomic orbital is formed by mixing a s orbital
with a p orbital. This creates two new orbitals called sp
hybrid orbitals (the sp is used to designate the kinds of
orbitals from which the hybrid was formed).
Notice that each of the hybrid orbitals has the same shape - each
has one large lobe and another much smaller lobe. The large lobe extends
further from the nucleus than either the s or p orbital from which the
hybrid orbital was formed. This allows the hybrid orbital to overlap
more effectively with an orbital on another atom when a bond is formed.
In general, the greater the overlap of two orbitals, the stronger the
bond.
Another point to notice is that the large lobes of the two sp
hybrid orbitals point in opposite directions - that is, they are 180o
apart.
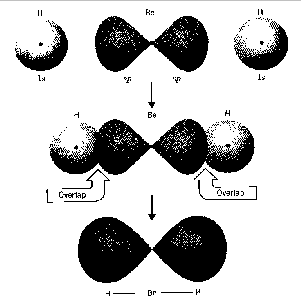
Let's look at a specific example, the linear beryllium hydride
molecule, BeH2, as it would be formed in the gas phase.
The orbital diagram for the valence shell of beryllium is

Note that the 2s orbital is filled and the three 2p orbitals are
empty. For bonds to form at a 180o angle between beryllium
and the two hydrogen atoms, two conditions must be met:(1) the two
orbitals that beryllium uses to for the Be-H bonds must be aligned
oppositely at 180o, and (2) each of the beryllium orbitals
must contain only one electron. The reason for the first requirement is
obvious. The reason for the second is that each bond is that each bond
must contain two electrons, one from the beryllium and one from the
hydrogen. The net effect of all this is that when the Be-H bonds form,
the electrons from the beryllium unpaired, and the resulting half-filled
s and p atomic orbitals become hybridized.
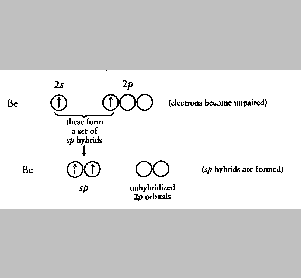
|