Nylon-66
This page is all about how to make nylon. We're telling you this as if you
can't read the title for yourself. To start off, nylon is made by a reaction
which is a step-growth
polymerization, and a
condensation polymerization. Nylons are made from diacids and diamines. If
you want to see what adipic acid and hexamethylene diamine look like in 3-D,
.
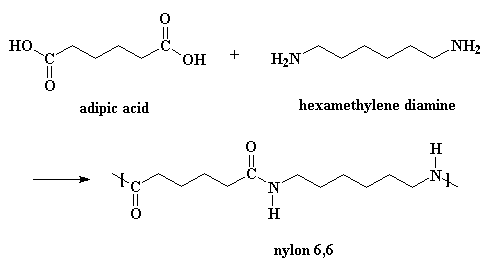
Of course, there are a few more details to the reaction than you see up
there in that little picture.
To make nylon 6,6 on doesn't need a catalyst, but acids do catalyze
the reaction, and wouldn't you know it, one of the monomers is itself an acid. A
little reaction happens between two adipic acid molecules. One will donate a
proton to a the carbonyl oxygen of another.
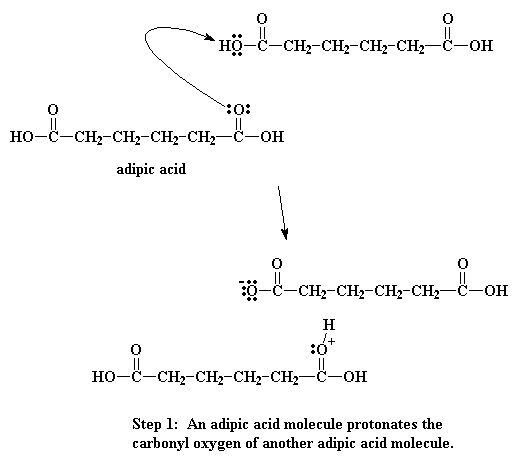
When this oxygen is protonated the carbonyl oxygen becomes much more
vulnerable to attack by the nitrogen of our diamine. This happens because our
protonated oxygen bears a positive charge. Oxygen does not like to have a
positive charge. So it pulls the electrons it shares with the carbonyl toward
itself. This leaves the carbonyl carbon lacking electrons, and ready for the
amine nitrogen to donate a pair to it:
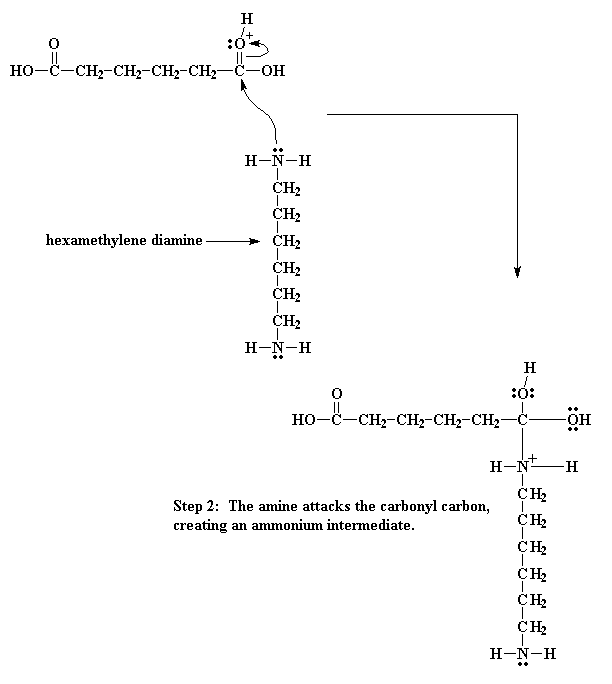
Then the electrons play musical chairs. One of the electron pairs form the
carbonyl double bond shifts entirely to the oxygen, taking care of the problem
of the positive charge at that atom, but now our nitrogen has a positive charge.
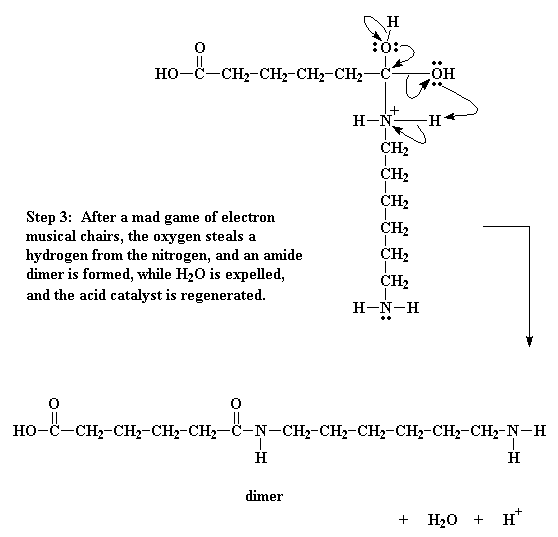
So then we get an even more elaborate game of electron musical chairs. The
electrons from the hydrogen oxygen bond go back to the oxygen, freeing the
proton, regenerating the acid catalyst. Then the carbonyl oxygen shares its
newly regained electrons with the carbon atoms, regenerating the carbonyl double
bond.
Of course, this isn't enough. The oxygen of the hydroxyl group decides to
do a little bit of electron shuffling of its own. It takes the pair it shares
with the carbon and hogs them to itself, severing the bond between it and the
carbon. It then donates a pair of electrons to a hydrogen connected to the
nitrogen.
That gets this hydrogen thinking. As it now shares a pair of electrons
with the oxygen, it sees no need to keep the pair it shares with the nitrogen,
so it lets go of that pair, giving it over to the nitrogen. This shift of
electrons breaks the bond between the hydrogen and the nitrogen, and gets rid of
the positive charge on that nitrogen. It splits off H2O, and
generates the amide-containing dimer.
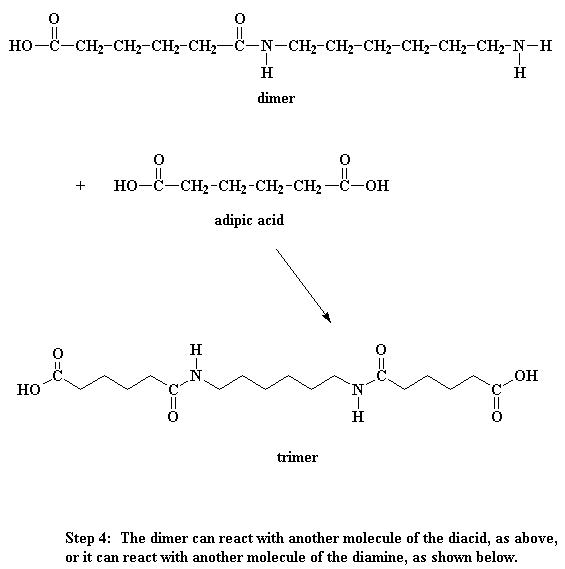
So what does this dimer do? Look close, and you'll see that it has an acid
group at one end, and an amine group at the other. This means that it can react
with a molecule of the diacid, or a molecule of the diamine. Either way, you get
a trimer.
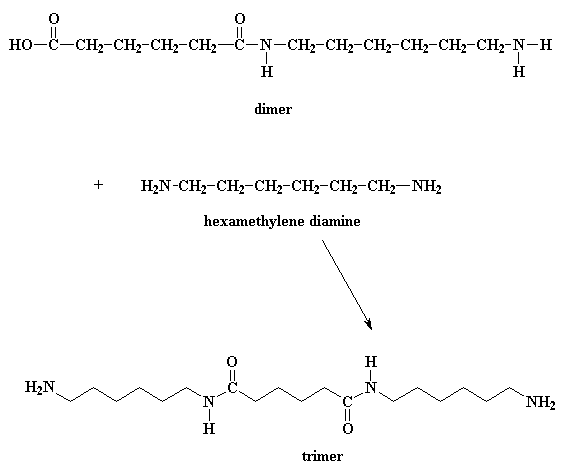
Wanna know a little secret? Our dimer can also react with other dimers, to
make a tetramer if it wants to. Or it can react with a trimer to form a pentamer,
and it can also react with bigger oligomers. Eventually, when this happens,
dimers will grow into trimers, tetramers, and bigger oligomers, and these big
oligomers will react with each other, to form even bigger oligomers. This keeps
happening until they become big enough to be called polymers.
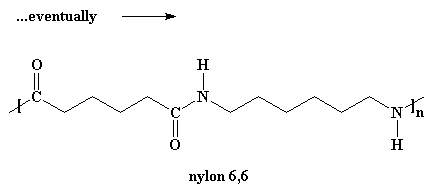
For the molecules to grow big enough to be called polymers, we have to do
this reaction under a vacuum. When we do this, all that by-product water will
evaporate and get sucked away. We need to get rid of the water because of a
little rule called Le
Chatelier's Principle.
Now remember how at the beginning of this little lesson I said that the
reaction doesn't need an acid catalyst to take place? The reason we know
this is that near the end of the polymerization, when there aren't many acid
groups left to be catalysts, the reaction still goes on. You see, the amine can
react with the unprotonated carboxylic acids. If this were not so, high
molecular weight nylon 66 could not be made without an external catalyst,
because the reaction would stop at higher conversions, when there aren't many
acid groups left to be catalysts.
Wanna know something else?
Nylons can also be made from a diamine and a diacid chloride:
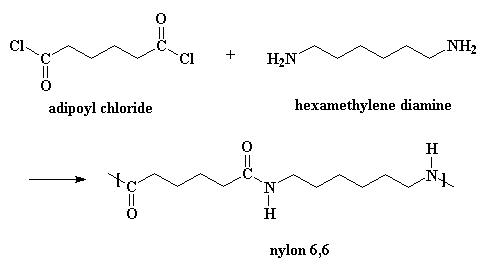
This reaction goes by the same mechanism, but you need to add a little bit
of acid to act as a catalyst. (When you make nylon the other way, adipic acid
acts as the catalyst.) Also, it produces HCl gas as a byproduct rather than
water. If you want to see a movie of this method
|

