Depending on its processing and thermal history, it may exist both as an
amorphous (transparent) and as a semi-crystalline
(opaque and white) material. Its monomer can be synthesized by the
esterification reaction between
terephthalic acid and
ethylene glycol with water as a byproduct, or the
transesterification reaction between
ethylene glycol and
dimethyl terephthalate with
methanol as
a byproduct. Polymerization is through a polycondensation reaction of the
monomers (done immediately after esterification/transesterification) with
ethylene glycol as the byproduct (the ethylene glycol is recycled in
production).
The majority of the world's PET production is for synthetic fibers (in excess
of 60%) with bottle production accounting for around 30% of global demand. In
discussing textile applications, PET is generally referred to as simply "polyester"
while "PET" is used most often to refer to packaging applications.
Some of the trade names of PET products are Dacron, Diolen,
Terylene, andTrevira
fibers,
Cleartuf, Eastman PET and Polyclear bottle resins,
Hostaphan, elinex, and Mylar
films, and Arnite, Ertalyte, Impet,Rynite and
Valox
injection molding resins. The
polyester
Industry makes up about 18% of world polymer production and is third after
polyethylene (PE) and
polypropylene (PP).
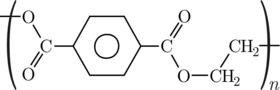
Chemical structure of polyethylene terephthalate
|
Contents
-
1 Uses
-
2 Intrinsic
viscosity
-
3 Drying
-
4 Copolymers
-
5 Crystals
-
6 Degradation
-
7 Antimony
-
8
Re-crystallization
-
9 Processing
equipment
-
10 See also
-
11 References
-
12 External
links
|
Uses

soft drink
bottle
PET can be semi-rigid to rigid, depending on its thickness, and is very
lightweight. It makes a good gas and fair moisture barrier, as well as a good
barrier to
alcohol (requires additional "Barrier" treatment) and
solvents. It
is strong and
impact-resistant. It is naturally colorless with high transparency.
When produced as a thin film (often known by the tradename
Mylar), PET is often
coated
with
aluminium to reduce its permeability, and to make it reflective and opaque.
PET bottles are excellent barrier materials and are widely used for
soft drinks, (see
carbonation). PET or Dacron is also used as a
thermal insulation layer on the outside of the
International Space Station as seen in an episode of
Modern Marvels "Sub Zero". For certain specialty
bottles, PET sandwiches an additional
polyvinyl alcohol to further reduce its
oxygen
permeability.
When filled with
glass
particles or
fibers, it becomes significantly
stiffer and more durable. This glass-filled plastic, in a semi-crystalline
formulation, is sold under the tradename Rynite, Arnite,
Hostadur, and Crastin.

Sails
are usually made of Dacron, a brand of PET fiber; colorful
lightweight
spinnakers are usually made of
nylon.
While most thermoplastics can, in principle, be recycled, PET bottle
recycling
is more practical than many other plastic applications. The primary reason is
that plastic carbonated soft drink bottles and
water
bottles are almost exclusively PET which makes them more easily identifiable
in a recycle stream. PET has a
resin identification code of 1. PET, as with many plastics, is also an
excellent candidate for thermal recycling (incineration) as it is composed of
carbon, hydrogen and oxygen with only trace amounts of catalyst elements (no
sulphur) and has the energy content of soft coal.
One of the uses for a recycled PET bottle is for the manufacture of
polar
fleece material. It can also make fiber for polyester products.
PET was patented in
1941 by the Calico Printers' Association of
Manchester.
The PET bottle was patented in
1973.
Intrinsic viscosity
One of the most important characteristics of PET is referred to as I.V. (intrinsic
viscosity).
The I.V. of the material, measured in
deciliters per gram (dl/g) is dependent upon the length of its polymer
chains. The longer the chains, the stiffer the material, and therefore the
higher the I.V. The average chain length of a particular batch of resin can be
controlled during
polymerization.
An I.V. of about:
- 0.60 dl/g: Would be appropriate for
fibre
- 0.65 dl/g: Film
- 0.76-0.84 dl/g: Bottles
- 0.85 dl/g: Tire
cord
Drying
PET is
hygroscopic, meaning that it naturally absorbs water from its surroundings.
However, when this 'damp' PET is then heated a chemical reaction known as
hydrolysis
takes place between the water and the PET which reduces its molecular weight
(IV) and its physical properties. This means that before the resin can be
processed in a molding machine, as much moisture as possible must be removed
from the resin. This is achieved through the use of a
desiccant
or dryers before the PET is fed into the processing equipment.
Inside the dryer, hot dry air is pumped into the bottom of the hopper
containing the resin so that it flows up through the pellets, removing moisture
on its way. The hot wet air leaves the top of the hopper and is first run
through an after-cooler, because it is easier to remove moisture from cold air
than hot air. The resulting cool wet air is then passed through a
desiccant
bed. Finally the cool dry air leaving the desiccant bed is re-heated in a
process heater and sent back through the same processes in a closed loop.
Typically residual moisture levels in the resin must be less than 40 parts per
million (parts of water per million parts of resin, by weight) before
processing. Dryer residence time should not be shorter than about four hours.
This is because drying the material in less than 4 hours would require a
temperature above 160 �C, at which level
hydrolysis
would begin inside the pellets before they could be dried out.
Copolymers
In addition to pure (homopolymer)
PET, PET modified by
copolymerization is also available.
In some cases, the modified properties of copolymer are more desirable for a
particular application. For example,
cyclohexane dimethanol (CHDM) can be added to the polymer backbone in place
of
ethylene glycol. Since this building block is much larger (6 additional
carbon atoms) than the ethylene glycol unit it replaces, it does not fit in with
the neighbouring chains the way an ethylene glycol unit would. This interferes
with crystallization and lowers the polymer's
melting temperature. Such PET is generally known as PETG (EastmanChemical
and SKchemicals are the only two manufacturers).
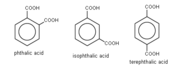
Replacing terephthalic acid (right) with isophthalic acid (center)
creates a kink in the PET chain, interfering with
crystallization and lowering the polymer's
melting point.
Another common modifier is isophthalic acid, replacing some of the 1,4-(para-)
linked
terephthalate units. The 1,2-(ortho-) or 1,3-(meta-)
linkage produces an angle in the chain, which also disturbs crystallinity.
Such copolymers are advantageous for certain moulding applications, such as
thermoforming, which is used for example to make tray or blister packagings
from PETG film, or
PETG sheet. On the other hand, crystallization is important in other
applications where mechanical and dimensional stability are important, such as
seat belts.
For PET bottles, the use of small amounts of CHDM or other comonomers can be
useful: if only small amounts of comonomers are used, crystallization is slowed
but not prevented entirely. As a result, bottles are obtainable via stretch
blow
molding ("SBM"), which are both clear and crystalline enough to be an
adequate barrier to aromas and even gases, such as carbon dioxide in carbonated
beverages.
Crystals
Crystallization occurs when polymer chains fold up on themselves in a
repeating, symmetrical pattern. Long polymer chains tend to become entangled on
themselves, which prevents full crystallization in all but the most carefully
controlled circumstances. PET is no exception to this rule; 60% crystallization
is the upper limit for commercial products, with the exception of polyester
fibers.
PET in its natural state is a crystalline resin. Clear products can be
produced by rapidly cooling molten polymer to form an
amorphous solid. Like
glass, amorphous
PET forms when its molecules are not given enough time to arrange themselves in
an orderly fashion as the melt is cooled. At room temperature the molecules are
frozen in place, but if enough heat energy is put back into them, they begin to
move again, allowing crystals to
nucleate
and grow. This procedure is known as
solid-state crystallization.
Like most materials, PET tends to produce many small
crystallites when crystallized from an amorphous solid, rather than forming
one large single crystal. Light tends to scatter as it crosses the boundaries
between crystallites and the amorphous regions between them. This scattering
means that crystalline PET is opaque and white in most cases.
Fiber
drawing is among the few industrial processes that produces a nearly
single-crystal product.
Degradation
PET is subject to various types of degradations during processing. The main
degradations that can occur are hydrolytic, thermal and probably most important
thermal oxidation. When PET degrades, several things happen: discoloration,
chain scissions resulting in reduced molecular weight, formation of
acetaldehyde and
cross-links
("gel" or "fish-eye" formation). Discoloration is due to the formation of
various chromophoric systems following prolonged thermal treatment at elevated
temperatures. This becomes a problem when the optical requirements of the
polymer are very high like for example in packaging applications. Acetaldehyde
is normally a colorless gas with a fruity smell. It forms naturally in fruit,
but it can cause an off-taste in bottled water. Acetaldehyde forms in PET
through the "abuse" of the material. High temperatures (PET decomposes above 300
�C or 570 �F), high pressures, extruder speeds (excessive shear flow raises
temperature) and long barrel residence times all contribute to the production of
acetaldehyde. When acetaldehyde is produced, some of it remains dissolved in the
walls of a container and then
diffuses
into the product stored inside, altering the taste and aroma. This is not such a
problem for non-consumables such as shampoo, for fruit juices, which already
contain acetaldehyde or for strong-tasting drinks, such as
soft drinks.
For bottled water, low acetaldehyde content is quite important, because if
nothing masks the aroma, even extremely low concentrations (10-20 parts per
billion parts of resin, by weight) of acetaldehyde can produce an off-taste. The
thermal and thermooxidative degradation results in poor processability
characteristics and performance of the material.
One way to alleviate this is to use a
copolymer.
Comonomers such as CHDM or
isophthalic acid lower the
melting temperature and reduces the degree of crystallinity of PET
(especially important when the material is used for bottle manufacturing). Thus
the resin can be plastically formed at lower temperatures and/or with lower
force. This helps to prevent degradation, reducing the acetaldehyde content of
the finished product to an acceptable (that is, unnoticeable) level. See
copolymers, above. Other ways to improve the stability of the polymer is by
using stabilizers, mainly antioxidants such as phosphites. Recently, molecular
level stabilization of the material using nanostructured chemicals has also been
considered.
Antimony
Antimony trioxide (Sb2O3) is a
catalyst that is often used in the production of PET. It remains in the
material and can thus in principle migrate out into food and drinks. Although
antimony trioxide is of low toxicity, its presence is still of concern. The
Swiss Federal Office of Public Health investigated the amount of antimony
migration, comparing waters bottled in PET and glass: the antimony
concentrations of the water in PET bottles was higher, but still well below the
allowed maximal
concentrations.[1]
(report available in German and French only) The Swiss Federal Office of Public
Health concluded that small amounts of antimony migrate from the PET into
bottled water, but that the health risk of the resulting low concentrations is
negligible (1% of the "tolerable daily intake" determined by the
WHO). A later (2006) but more widely publicized study by a group of
geochemists at the University of Heidelberg headed by William Shotyk found
similar amounts of antimony in water in PET bottles.[2]
The most recent WHO risk assessment for antimony in drinking water can be
found here:
[3]
Re-crystallization

PETE has
SPI
resin ID code 1
PET can be used to explore the
crystallization of
amorphous solids. The
resin identification code can be used to verify the type of plastic it is
made of: many plastic beverage bottles have the letters PET or PETE and a code
of 1 on the bottom, near the center. When a flame is held several inches below
the bottle and slowly brought closer, part of the material will visibly change.
This happens because high temperatures melt the PET. This releases the tension
that was frozen in during the blow molding process and the polymer chains will
shift to a more relaxed and disordered state, which results in shrinkage of the
softened area. Because of the decreased order of the polymer chains, there are
now fewer crystal nuclei. Consequently, when the
crystallites re-form upon cooling they grow larger than the original
crystallites in the bottle wall. Because the new crystallites are larger than
the wave length of light, they will now cause light to scatter, giving the
material an opaque white appearance.
Processing equipment
There are two basic molding methods, one-step and two-step. In two-step
molding, two separate machines are used. The first machine injection molds the
preform. The preform looks like a test tube. The bottle-cap threads are already
molded into place, and the body of the tube is significantly thicker, as it will
be inflated into its final shape in the second step using
stretch-blow molding.
In the second process, the preforms are heated rapidly and then inflated
against a two-part mold to form them into the final shape of the bottle.
Preforms (uninflated bottles) are now also used as containers for candy.
In
one-step machines, the entire process from raw material to finished
container is conducted within one machine, making it especially suitable for
molding non-standard shapes (custom molding), including
jars, flat oval,
flask shapes etc. Its greatest merit is the reduction in space, product handling
and energy, and far higher visual quality than can be achieved by the two-step
system.

