VSEPR Theory:
The Shapes of Molecules
One of the important ways that molecular compounds differ from
ionic compounds is in their structures. Ionic bonding is
nondirectional in the sense that, at a given distance, an ion will
attract others of the opposite sign with the same force regardless of
where these other ions are located around the ion in question - there is
no preferred direction. Because of this, the way ions arrange themselves
in an ionic solid is determined just by the tendency to maximize
attractions between ions of opposite charge and to minimize repulsions
between like-charged ions.
In molecular compounds, quite a different situation exists.
Covalent bonds are highly directional. That is, for a given
central atom in a molecule there are preferred orientations for the
atoms attached to it, because covalent bonds are not formed with equal
ease in all directions. As a result, in polyatomic molecules the atoms
remain in the same relative orientation, regardless of whether the
substance is a solid, liquid or a gas and we can say that the molecules
have a definite structure or shape.
The shapes of molecules are very important because many of their
physical and chemical properties depend upon the three-dimensional
arrangements of their atoms. For example, the functioning of enzymes,
which are substances that control how fast biochemical reactions occur,
requires that there be a very precise fit between one molecule and
another. Even slight alterations in molecular geometry can destroy this
fit and deactivate the enzyme, which in turns prevents the biochemical
reaction involved from occurring. Nerve poisons work this way.
There is a very simple theory that is remarkably effective in
predicting the shapes of molecules formed by the representative
elements. It is called the valence shell electron pair repulsion
theory (VSEPR theory, for short). The theory is based on the idea
that valence shell electron pairs, being negatively charged, stay as far
part from each other as possible so that repulsions between them at a
minimum.
Consider the BeCl2 molecule. We've seen that its Lewis
structure is
.. ..
:Cl:Be:Cl:
.. ..
But how are these atoms arranged? Is BeCl2 linear or
is it nonlinear, that is, do the atoms lie in a straight line, or do
they form some angels less than 180o?

According to VSEPR theory, we can predict the shape of a molecule
by looking at the electron pairs in the valence shell of the central
atom. For BeCl2, there are two pairs of electrons around the
central beryllium atom. The question is, how can they locate themselves
to be as far apart as possible? The answer is that the minimum
repulsions will occur when the electron pairs are on opposite sides of
the nucleus. (180o apart).
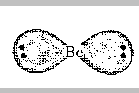
In order for the electrons to be in the Be-Cl bonds, the
Cl atoms must be placed where the electrons are; the result is that we
predict that a BeCl2 molecule should be linear.
Cl-Be-Cl
Another example will be the BCl3 molecule. Its Lewis
structure is
..
:Cl:
.. .. ..
:Cl:B:Cl:
.. ..
Here, the central atom, B, has three electron pairs. What
arrangement will lead to minimum repulsions? The electron pairs will be
as far apart as possible when they are located at the corners of a
triangle with the boron in the centre.

When we attach the Cl atoms we obtain a triangular molecule.

This shape is planar triangular, because all four atoms lie in
the equatorial plane.
In some molecules, all of the central pairs of the central atom
are not bonding pairs. These unbonded pairs are actually unshared
electron pairs - also called lone pairs - and these affect the geometry
of the molecule. An example is SnCl2.
.. .. ..
:Cl:Sn:Cl:
.. ..
There are three pairs of electrons around the central Sn atom -
the two in the bonds plus the lone pair. As in BCl3 , the
mutual repulsions of the three pairs will place them at the corners of a
triangle.
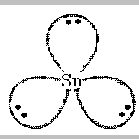
Adding on the two chlorine atoms gives
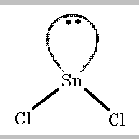
This is not a triangular shape, even though that is how the
electron pairs are arranged. Molecular shape describes the arrangements
of atoms, not the arrangement of electron pairs. Therefore, this shape
is described as being bent.
|

