About Temperature
Contents
What is Temperature
The Development of Thermometers and Temperature Scales
Heat and Thermodynamics
The Kinetic Theory
Thermal Radiation
3 K - The Temperature of the Universe
Summary
Acknowledgments
References
What is Temperature?
In a qualitative manner, we can describe the temperature of an
object as that which determines the sensation of warmth or coldness felt from
contact with it.It is easy to demonstrate that when two
objectsof the same material are placed together (physicists say when they are
put in thermal contact), the object with the higher temperature cools while the
cooler object becomes warmer until a point is reached after which no more change
occurs, and to our senses, they feel the same. When the thermal changes have
stopped, we say that the two objects (physicists define them more rigorously as
systems) are in thermal equilibrium . We can then define the temperature
of the system by saying that the temperature is that quantity which is the same
for both systems when they are in thermal equilibrium.
If we experiment further with more than two systems, we find
that many systems can be brought into thermal equilibrium with each other;
thermal equilibrium does not depend on the kind of object used. Put more
precisely,
if two systems are separately in thermal equilibrium with a
third, then they must also be in thermal equilibrium with each other,
and they all have the same temperature regardless of the kind
of systems they are.
The statement in italics, called the zeroth law of
thermodynamics may be restated as follows:
If three or more systems are in thermal contact with each
other and all in equilibrium together, then any two taken separately are in
equilibrium with one another. (quote from T. J. Quinn's monograph
Temperature)
Now one of the three systems could be an instrument calibrated to
measure the temperature - i.e. a thermometer. When a calibrated thermometer is
put in thermal contact with a system and reaches thermal equilibrium, we then
have a quantitative measure of the temperature of the system. For example, a
mercury-in-glass clinical thermometer is put under the tongue of a patient and
allowed to reach thermal equilibrium in the patient's mouth - we then see by how
much the silvery mercury has expanded in the stem and read the scale of the
thermometer to find the patient's temperature.
What is a Thermometer?
A thermometer is an instrument that measures the temperature of a
system in a quantitative way. The easiest way to do this is to find a substance
having a property that changes in a regular way with its temperature. The most
direct 'regular' way is a linear one:
t(x) = ax + b,
where t is the temperature of the substance and changes as the
property x of the substance changes. The constants a and b depend on the
substance used and may be evaluated by specifying two temperature points on the
scale, such as 32� for the freezing point of water and 212� for its boiling
point.
For example, the element mercury is liquid in the temperature
range of -38.9� C to 356.7� C (we'll discuss the Celsius � C scale later). As a
liquid, mercury expands as it gets warmer, its expansion rate is linear and can
be accurately calibrated.
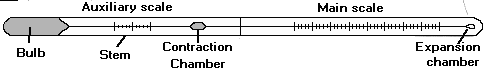
The mercury-in-glass thermometer illustrated in the above
figure contains a bulb filled with mercury that is allowed to expand into a
capillary. Its rate of expansion is calibrated on the glass scale.
|

