The Development of Thermometers and Temperature Scales
The historical highlights in the development of thermometers
and their scales given here are based on "Temperature" by T. J. Quinn and "Heat"
by James M. Cork.
One of the first attempts to make a standard temperature scale
occurred about AD 170, when
Galen, in his medical writings, proposed a standard "neutral" temperature
made up of equal quantities of boiling water and ice; on either side of this
temperature were four degrees of heat and four degrees of cold, respectively.
The earliest devices used to measure the temperature were called thermoscopes.
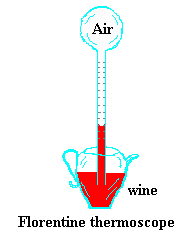 They
consisted of a glass bulb having a long tube extending downward into a container
of colored water, although
Galileo in 1610 is supposed to have used wine. Some of the air in the bulb
was expelled before placing it in the liquid, causing the liquid to rise into
the tube. As the remaining air in the bulb was heated or cooled, the level of
the liquid in the tube would vary reflecting the change in the air temperature.
An engraved scale on the tube allowed for a quantitative measure of the
fluctuations. They
consisted of a glass bulb having a long tube extending downward into a container
of colored water, although
Galileo in 1610 is supposed to have used wine. Some of the air in the bulb
was expelled before placing it in the liquid, causing the liquid to rise into
the tube. As the remaining air in the bulb was heated or cooled, the level of
the liquid in the tube would vary reflecting the change in the air temperature.
An engraved scale on the tube allowed for a quantitative measure of the
fluctuations.
The air in the bulb is referred to as the thermometric medium, i.e.
the medium whose property changes with temperature.
In 1641, the first sealed thermometer that used liquid rather than air as the
thermometric medium was developed for Ferdinand II, Grand Duke of Tuscany. His
thermometer used a sealed alcohol-in-glass device, with 50 "degree" marks on its
stem but no "fixed point" was used to zero the scale. These were referred to as
"spirit" thermometers.
Robert Hook, Curator of the Royal Society, in 1664 used a red dye in the
alcohol . His scale, for which every degree represented an equal increment of
volume equivalent to about 1/500 part of the volume of the thermometer liquid,
needed only one fixed point. He selected the freezing point of water. By scaling
it in this way, Hook showed that a standard scale could be established for
thermometers of a variety of sizes. Hook's original thermometer became known as
the standard of Gresham College and was used by the Royal Society until 1709.
(The first intelligible meteorological records used this scale).
In 1702, the astronomer Ole Roemer of Copenhagen based his scale upon
two fixed points: snow (or crushed ice) and the boiling point of water,
and he recorded the daily temperatures at Copenhagen in 1708- 1709 with this
thermometer.
It was in 1724 that Gabriel Fahrenheit, an instrument maker of D�anzig and
Amsterdam, used mercury as the thermometric liquid. Mercury's thermal expansion
is large and fairly uniform, it does not adhere to the glass, and it remains a
liquid over a wide range of temperatures. Its silvery appearance makes it easy
to read.
Fahrenheit described how he calibrated the scale of his mercury thermometer:
"placing the thermometer in a mixture of sal ammoniac or sea salt, ice, and
water a point on the scale will be found which is denoted as zero. A second
point is obtained if the same mixture is used without salt. Denote this
position as 30. A third point, designated as 96, is obtained if the
thermometer is placed in the mouth so as to acquire the heat of a healthy
man." (D. G. Fahrenheit,Phil. Trans. (London) 33, 78, 1724)
On this scale, Fahrenheit measured the boiling point of water to be 212.
Later he adjusted the freezing point of water to 32 so that the interval between
the boiling and freezing points of water could be represented by the more
rational number 180. Temperatures measured on this scale are designated as
degrees Fahrenheit (� F).
In 1745, Carolus Linnaeus of Upsula, Sweden, described a scale in which the
freezing point of water was zero, and the boiling point 100, making it a
centigrade (one hundred steps) scale. Anders Celsius (1701-1744) used the
reverse scale in which 100 represented the freezing point and zero the boiling
point of water, still, of course, with 100 degrees between the two defining
points.
In 1948 use of the Centigrade scale was dropped in favor of a new scale using
degrees Celsius (� C). The Celsius scale is defined by the
following two items that will be discussed later in this essay:
(i) The triple point of water is defined to be 0.01� C.
(ii) A degree Celsius equals the same temperature change as a degree on the
ideal-gas scale.
On the Celsius scale the boiling point of water at standard atmospheric
pressure is 99.975 C in contrast to the 100 degrees defined by the Centigrade
scale.
To convert from Celsius to Fahrenheit: multiply by 1.8 and add 32.
� F = 1.8� C + 32
� K = � C + 273.
(Or, you can get
someone else
to do it for you!)
In 1780, J. A. C. Charles, a French physician, showed that for the same
increase in temperature, all gases exhibited the same increase in volume.
Because the expansion coefficient of gases is so very nearly the same, it is
possible to establish a temperature scale based on a single fixed point rather
than the two fixed- point scales, such as the Fahrenheit and Celsius scales.
This brings us back to a thermometer that uses a gas as the thermometric medium.
 In a
constant volume gas thermometer a large bulb B of gas, hydrogen for example,
under a set pressure connects with a mercury-filled "manometer" by means of a
tube of very small volume. (The Bulb B is the temperature-sensing portion and
should contain almost all of the hydrogen). The level of mercury at C may be
adjusted by raising or lowering the mercury reservoir R. The pressure of the
hydrogen gas, which is the "x" variable in the linear relation with temperature,
is the difference between the levels D and C plus the pressure above D. In a
constant volume gas thermometer a large bulb B of gas, hydrogen for example,
under a set pressure connects with a mercury-filled "manometer" by means of a
tube of very small volume. (The Bulb B is the temperature-sensing portion and
should contain almost all of the hydrogen). The level of mercury at C may be
adjusted by raising or lowering the mercury reservoir R. The pressure of the
hydrogen gas, which is the "x" variable in the linear relation with temperature,
is the difference between the levels D and C plus the pressure above D.
P. Chappuis in 1887 conducted extensive studies of gas thermometers with
constant pressure or with constant volume using hydrogen, nitrogen, and carbon
dioxide as the thermometric medium. Based on his results, the Comit�
International des Poids et Mesures adopted the constant-volume hydrogen scale
based on fixed points at the ice point (0� C) and the steam point (100� C) as
the practical scale for international meteorology.
Experiments with gas thermometers have shown that there is very little
difference in the temperature scale for different gases. Thus, it is possible to
set up a temperature scale that is independent of the thermometric medium if it
is a gas at low pressure. In this case, all gases behave like an "Ideal Gas" and
have a very simple relation between their pressure, volume, and temperature:
pV= (constant)T.
This temperature is called the thermodynamic temperature and is now
accepted as the fundamental measure of temperature. Note that there is a
naturally-defined zero on this scale - it is the point at which the pressure of
an ideal gas is zero, making the temperature also zero. We will continue a
discussion of "absolute zero" in a later section. With this as one point on the
scale, only one other fixed point need be defined. In 1933, the International
Committee of Weights and Measures adopted this fixed point as the
triple point of water , the
temperature at which water, ice, and water vapor coexist in equilibrium); its
value is set as 273.16. The unit of temperature on this scale is called the
kelvin, after Lord Kelvin
(William Thompson), 1824-1907, and its symbol is K (no degree symbol used).
To convert from Celsius to Kelvin, add 273.
K = � C + 273.
Thermodynamic temperature is the fundamental temperature; its unit is the
kelvin which is defined as the fraction 1/273.16 of the thermodynamic
temperature of the triple point of water.
Sir William Siemens, in 1871, proposed a thermometer whose thermometric
medium is a metallic conductor whose resistance changes with temperature. The
element platinum does not oxidize at high temperatures and has a relatively
uniform change in resistance with temperature over a large range. The
Platinum Resistance Thermometer is now widely used as a thermoelectric
thermometer and covers the temperature range from about -260� C to 1235� C.
Several temperatures were adopted as
Primary reference points so as
to define the International Practical Temperature Scale of 1968. The
International Temperature Scale of 1990 was adopted by the International
Committee of Weights and Measures at its meeting in 1989. Between 0.65K and
5.0K, the temperature is defined in terms of the vapor pressure - temperature
relations of the isotopes of helium. Between 3.0K and the triple point of neon
(24.5561K) the temperature is defined by means of a helium gas thermometer.
Between the triple point of hydrogen (13.8033K) and the freezing point of silver
(961.78�K) the temperature is defined by means of platinum resistance
thermometers. Above the freezing point of silver the temperature is defined in
terms of the Planck radiation law.
T. J. Seebeck, in 1826, discovered that when wires of different metals are
fused at one end and heated, a current flows from one to the other. The
electromotive force generated can be quantitatively related to the temperature
and hence, the system can be used as a thermometer - known as a thermocouple.
The thermocouple is used in industry and many different metals are used -
platinum and platinum/rhodium, nickel-chromium and nickel-aluminum, for example.
The National Institute of Standards and Technology (NIST)
maintains databases for standardizing thermometers.
For the measurement of very low temperatures, the magnetic susceptibility of
a paramagnetic substance is used as the thermometric physical quantity. For some
substances, the magnetic susceptibility varies inversely as the temperature.
Crystals such as cerrous magnesium nitrate and chromic potassium alum have been
used to measure temperatures down to 0.05 K; these crystals are calibrated in
the liquid helium range.
This diagram and the last illustration in this text were taken from the Low
Temperature Laboratory, Helsinki University of Technology's
picture archive. For these
very low, and even lower, temperatures, the thermometer is also the mechanism
for cooling. Several
low-temperature laboratories conduct interesting applied and theoretical
research on how to reach the lowest possible temperatures and how work at these
temperatures may find application.
|

