Construction of the residue curve
Equation 3 and 4 were used to sketch the corresponding
residue curve for the three species. From the above information, we know that
these species have boiling points at 94.34 (cyclohexane), 93.49 (benzene) and
200.35oC (propylene glycol) at the pressure of 150 kPa, and an
azeotrope that boils at 91oC between the two more volatile species.
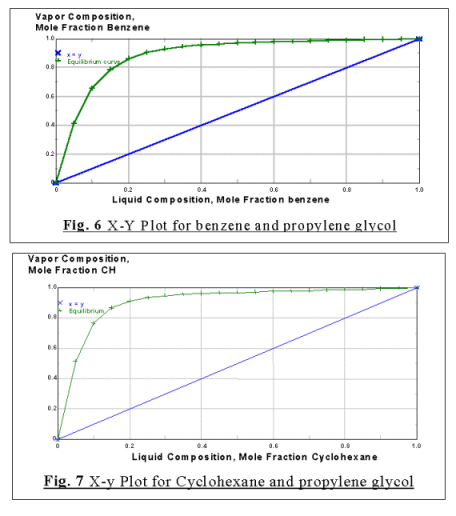
As were shown from Fig. 6 and Fig. 7 there were no new azeotropes formed between
the solvent 1,2-propanediol respectively with the another two component in the
feed.

Table 3 Result from computer simulation using
1,2-propanediol as solvent.
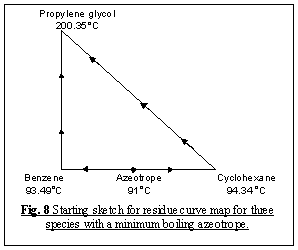
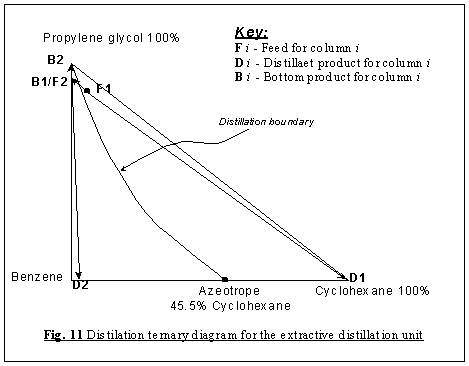
We then start to sketch our residue curve map by sketching
the triangular diagram in Fig. 8, and placing the arrows pointing from
the lower to higher temperatures around the edge. The corner points for benzene
and cyclohexane are single species point, and both are unstable nodes - all
residue curves leave. The corner point for propylene glycol is a single species
point which is a stable node - all residue curve enter. All three are nodes;
none are saddles, thus;
N1 = 3 and S1 = 0
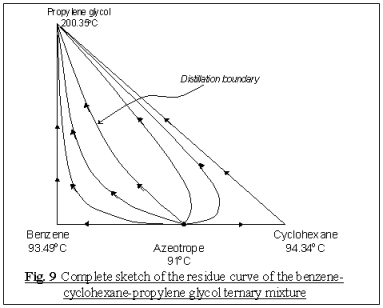
We then further assume that there will be no ternary
azeotrope been form among the three constituents, i.e.,
N3 = S3 = 0
The remaining steps here require the identification of the only
binary azeotrope that form between benzene and cyclohexane, to be either a node
or a saddle point. From equation 4:
4(0-0) + 2(N2 - S2) + (3-0) = 1
2(N2 - S2) = -2
N2 - S2 = -1
Thus, the only way we can satisfy the above equation is letting
N2 = 0 and S2 = 1, i.e. the binary azeotrope
is a saddle point, which directs the trajectories in another direction.
6.4 Column operation
The extractive distillation unit of this cyclohexane
production plant consists of two distillation columns , which we
can easily classify as direct sequence columns. The first column acts as
an extractive column where the solvent is introduced at the second stage of the
column, so that it will be present throughout the column and exits with the
bottoms. As were stated above, the solvent alters the natural volatility of the
binary mixture by forming hydrogen bonds with benzene and allowing it to be
recovered as the bottom product.
The bottom product of the first column will then fed to the
second column, i.e. the solvent recovery column, to undergo the normal
distillation to separate both the components for further usage, i.e. benzene
being recycled to the reactor for further conversion while solvent to the first
column for reuse. The main operation parameter of the distillation unit is shown
in Table 4.
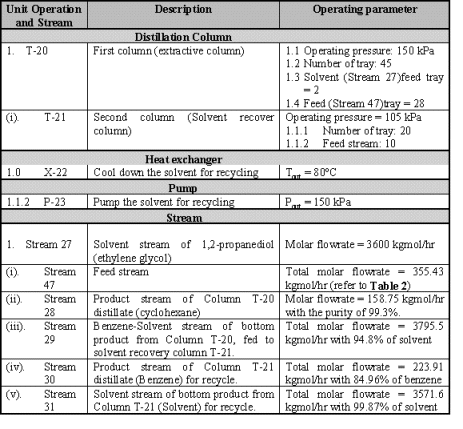
Table 4 Distillation unit summary
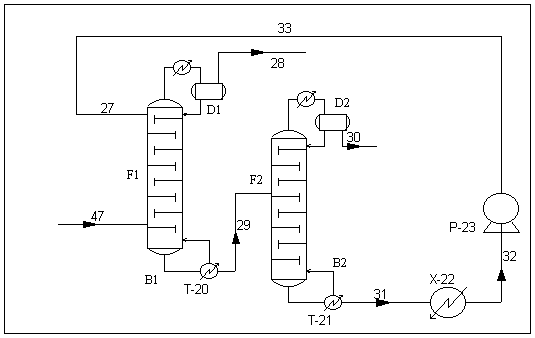
Fig. 10 Extractive distillation unit for cyclohexane
production plant
|