|
To understand why conjugation should cause bathochromic shifts in the
absorption maxima of chromophores, we need to look at the relative energy levels
of the pi-orbitals. When two double bonds are conjugated, the four p-atomic
orbitals combine to generate four pi-molecular orbitals (two are bonding and two
are antibonding). In a similar manner, the three double bonds of a conjugated
triene create six pi-molecular orbitals, half bonding and half antibonding. The
energetically most favorable π __> π* excitation occurs from
the highest energy bonding pi-orbital (HOMO) to the lowest energy
antibonding pi-orbital (LUMO).
The following diagram illustrates this excitation for an isolated double bond
(only two pi-orbitals). In each case the HOMO is colored blue and the LUMO is colored
magenta. Increased conjugation brings the HOMO and LUMO orbitals closer
together. The energy (ΔE) required to effect the electron promotion is therefore
less, and the wavelength that provides this energy is increased correspondingly
.
Many other kinds of conjugated pi-electron systems act as chromophores
and absorb light in the 200 to 800 nm region. These include unsaturated
aldehydes and ketones and aromatic ring compounds. A few examples are displayed
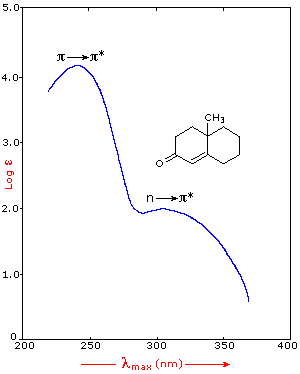
below. The spectrum of the unsaturated ketone (on the left) illustrates the
advantage of a logarithmic display of molar absorptivity. The π __> π*
absorption located at 242 nm is very strong, with an ε = 18,000. The weak n __> π*
absorption near 300 nm has an ε = 100.
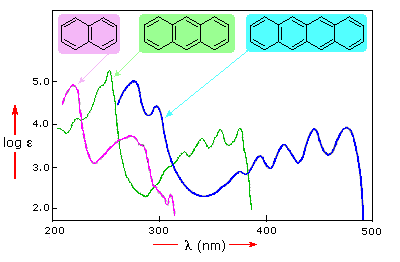
Benzene exhibits very strong light absorption near 180 nm (ε > 65,000) ,
weaker absorption at 200 nm (ε = 8,000) and a group of much weaker bands at 254
nm (ε = 240). Only the last group of absorptions are completely displayed
because of the 200 nm cut-off characteristic of most spectrophotometers. The
added conjugation in naphthalene, anthracene and tetracene causes bathochromic
shifts of these absorption bands, as displayed in the chart on the left below.
All the absorptions do not shift by the same amount, so for anthracene (green
shaded box) and tetracene (blue shaded box) the weak absorption is obscured by
stronger bands that have experienced a greater red shift. As might be expected
from their spectra, naphthalene and anthracene are colorless, but tetracene is
orange.
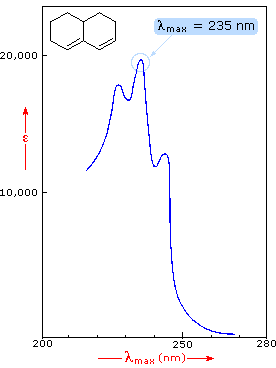
The spectrum of the bicyclic diene (above right) shows some vibrational fine
structure, but in general is similar in appearance to that of isoprene, Closer
inspection discloses that the absorption maximum of the more highly substituted
diene has moved to a longer wavelength by about 15 nm. This "substituent effect"
is general for dienes and trienes, and is even more pronounced for enone
chromophores.
|