Spin-Spin Interactions
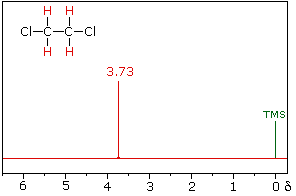
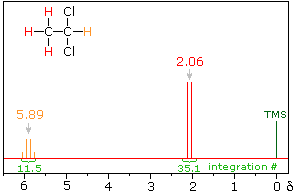
The nmr spectrum of 1,1-dichloroethane (below right) is more complicated
than we might have expected from the previous examples. Unlike its
1,2-dichloro-isomer (below left), which displays a single resonance signal
from the four structurally equivalent hydrogens, the two signals from the
different hydrogens are split into close groupings of two or more
resonances. This is a common feature in the spectra of compounds having
different sets of hydrogen atoms bonded to adjacent carbon atoms. The signal
splitting in proton spectra is usually small, ranging from fractions of a Hz
to as much as 18 Hz, and is designated as J (referred to as the
coupling constant). In the 1,1-dichloroethane example all the coupling
constants are 6.0 Hz.
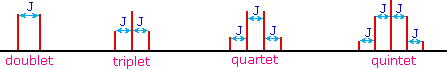
The splitting patterns found in various spectra are easily
recognized, provided the chemical shifts of the different sets of
hydrogen that generate the signals differ by two or more ppm. The
patterns are symmetrically distributed on both sides of the proton
chemical shift, and the central lines are always stronger than the outer
lines. The most commonly observed patterns have been given descriptive
names, such as doublet (two equal intensity signals), triplet
(three signals with an intensity ratio of 1:2:1) and quartet (a
set of four signals with intensities of 1:3:3:1). Four such patterns are
displayed in the following illustration. The line separation is always
constant within a given multiplet, and is called the coupling
constant (J). The magnitude of J, usually given in units of Hz, is
magnetic field independent.
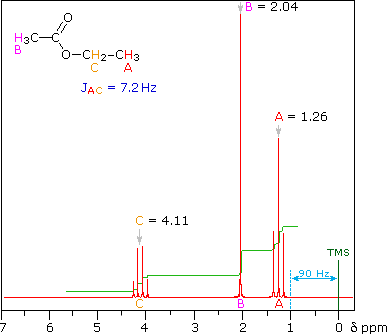
The splitting patterns shown above display the ideal or "First-Order"
arrangement of lines. This is usually observed if the spin-coupled
nuclei have very different chemical shifts (i.e. Δν is large compared to
J). If the coupled nuclei have similar chemical shifts, the splitting
patterns are distorted (second order behavior). In fact, signal
splitting disappears if the chemical shifts are the same. Two examples
that exhibit minor 2nd order distortion are shown below (both are taken
at a frequency of 90 MHz). The ethyl acetate spectrum on the left
displays the typical quartet and triplet of a substituted ethyl group.
The spectrum of 1,3-dichloropropane on the right demonstrates that
equivalent sets of hydrogens may combine their influence on a second,
symmetrically located set.
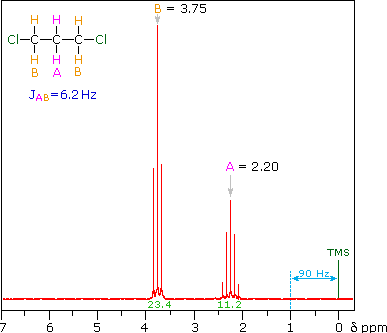
Even though the chemical shift difference between the A and B protons in
the 1,3-dichloroethane spectrum is fairly large (140 Hz) compared with
the coupling constant (6.2 Hz), some distortion of the splitting
patterns is evident. The line intensities closest to the chemical shift
of the coupled partner are enhanced. Thus the B set triplet lines
closest to A are increased, and the A quintet lines nearest B are
likewise stronger. A smaller distortion of this kind is visible for the
A and C couplings in the ethyl acetate spectrum.
|